Nototodarus hawaiiensis
Richard E. Young and Michael VecchioneIntroduction
Nototodarus hawaiiensis is often island-associated and captured over seamounts or island slopes but is also caught off the continental shelf of Australia. During the day, in Hawaiian waters, it has been observed sitting or swimming near the sea floor. At night its habits are unknown but it is not seen around surface night-lights. Maximum size known is about 250 mm ML, commonly reaches 150 mm ML (Dunning and Förch, 1998).

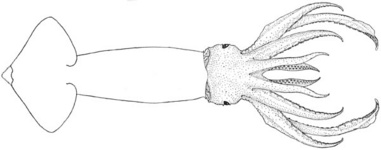

Figure. N. hawaiiensis. Top -Dorsal view of a female, 180 mm ML, Philippine waters.Drawing from Voss (1963). Bottom - Side view, 5-7 cm total length (estimate), in situ photograph while scuba diving off Hawaii. © 2017 Jeffrey Milisen.
Characteristics
- Arms (Dunning and Förch, 1998))
- Arms subequal, large.
- Each arm with 38-56 suckers
- largest arm suckers with 9 to 16 conical teeth interspersed with low flat truncated teeth, distal tooth significantly larger.
- Protective membranes and their supports of uniform height, not higher than suckers.
- Hectocotylus
- Both arms IV hectocotylized; entire right arm IV hectocotylized; base of left arm IV hectocotylized.
- Right arm IV with double series of slender conical papillae and expanded ventral protective membrane and supports opposite entire distal row of dorsal papillae (Dunning and Förch, 1998).
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window

Figure. Left - Oral-oblique view of a large sucker ring from arm III of N. hawaiiensis. Photograph by R. Young. Right - Drawing of an arm sucker ring of N. hawaiiensis, holotype. Drawing from Berry (1918).
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window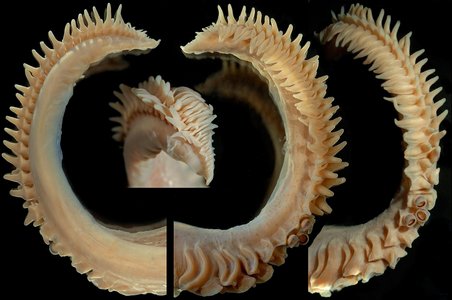

Figure. Hectocotylized arms IV of N. hawaiiensis. Top - Right arm IV. Symmetrical pair - dorsal (left) and ventral (right) views. Right - Oral view. Insert - Distal-oral view of the tip of right arm IV. Bottom - Left arm IV. Symmetrical pair - ventral (left) and dorsal (right) views. Insert - Oral view of arm base.
- Tentacles
- Large central tooth on distal margin of largest sucker rings.
- Largest club suckers with 14-18 large conical teeth interspersed with low plates.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window
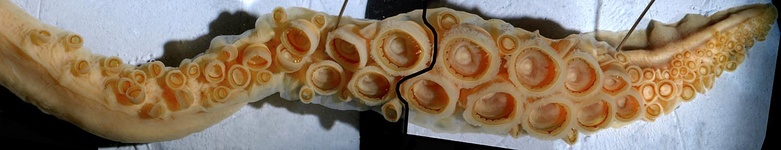
Figure. Oral views of the tentacular club of N. hawaiiensis. Top - i80 mm ML, Philippine waters. Drawing from Voss (1963). Bottom - 140 mm ML, Hawaiian waters. The twisted, preserved club was photographed from two angles to view the entire club. The two pictures were then joined; the black line shows the "joint." Photograph by R. Young.
- Head
- Beaks: Descriptions can be found here: Lower beak; upper beak.
- Beaks: Descriptions can be found here: Lower beak; upper beak.
- Funnel
- Funnel locking-apparatus with a simple, gently curved sulcus and sucal ridge. A detailed description can be found here.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window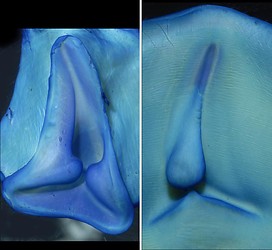

Figure. Funnel/mantle locking-apparatus of N. hawaiiensis, female, 135 mm ML, Hawaiian waters. Left - Frontal view of funnel component. Middle - Frontal view of mantle component. Right top - Frontal view of anterior tip of mantle component. Right bottom - Frontal view of the anterior tip of the funnel component. Photographs by R. Young.
- Funnel locking-apparatus with a simple, gently curved sulcus and sucal ridge. A detailed description can be found here.
- Pigmentation
- Narrow, dark pigment strip along the dorsal mantle midline
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window
Figure. Posterodorsal view of N. hawaiiensis, resting on the bottom of an aquarium, showing dorsal pigment strip. Photograph by R. Young.



Figure. Left - Oral-oblique view of the two largest sucker rings from the same tentacular club of N. hawaiiensis. Photograph by R. Young. Right - Drawing of a large club sucker ring, holotype. Drawing from Berry (1918).
Life History
Paralarvae
N. hawaiiensis paralarvae are characterized by being very broad relative to their length (ie, they are "fat"), having a very short proboscis with very large lateral suckers, and a distinctive band of chromatophores around the middle of the mantle by about 4.0 mm ML (Harman and Young, 1985).

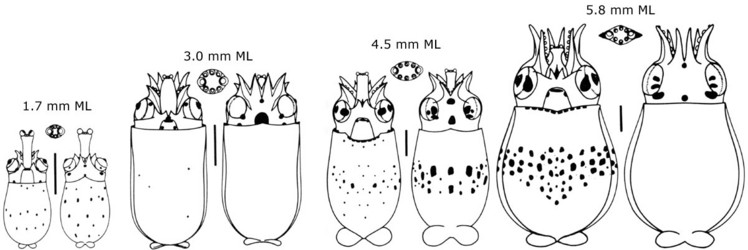
Figure. Ventral and dorsal views of paralarval stages of N. hawaiiensis. Inserts show proboscis tips. Chromatophore pattern is missing from the dorsal view of the 5.8 mm ML paralarva. Scale bar is 1 mm. Drawings from Harman and Young (1985).



Figure. N. hawaiiensis, late paralarvae/early juveniles. Left - Ventral and dorsal views of the first juvenile stage. Note the small tentacles. Scale bar is 1 mm. Drawings from Harman and Young (1985). Right - Dorsal view, in situ photograph while scuba diving. © 2014 Jeffrey Milisen.



Figure. N. hawaiiensis, late paralarvae/early juveniles. Left - Oral views (scanning electron microscopy) of the proboscis and proboscis suckers of N. hawaiiensis, 1.1 mm ML. A - Oral crown. B - Enlargement of proboscis tip. Photographs from Harman and Young (1985). Right - In situ photogaphs, several views. Note the thickness, and the position (well posterior to the cephalic cartilage) of the orange digestive gland. Image is a composite of under water photographs taken of a single paralarva (not the same as the paralarva in the previous Fig.) while scuba diving, © 2014 Jeffrey Milisen.
Adults
In Australian waters males generally mature by 130 mm ML and females by 150 mm ML (Dunning and Förch, 1998). In Hawaiian waters mature females have been found as small as 113 mm ML ; a mature female of 141 mm ML had 1500 eggs (amber color, 1.1 by 0.9 mm in size) in both oviducts (Young, 1995). Maximum size for a N. hawaiiensis from Hawaiian waters is 160 mm ML (Harman and Young, 1985).
Behavior
Submersible observations in Hawaiian waters (Young, 1995) indicated that during the day N. hawaiiensis are solitary and commonly rest on the ocean floor or swim just 10-20 cm above the bottom. When disturbed by the submersible, a resting squid resisted swimming and sometimes had to be physically prodded before swimming away. They often moved off slowly, vigorously flapping their relatively small fins (like a bird flapping its wings). When sufficiently disturbed, the squid abandoned this method of swimming and, with a strong beat of the fins and a simultaneous jet from the mantle, darted out of sight (see video below).Distribution
Type locality: Off Kahuku Point, Oahu, Hawaii, tropical North Pacific on bottom at 463-516 m, 1902.
Vertical distribution
N. hawaiiensis is primarily found over continental and island slope waters. It has been captured from depths of 160-700 m off Australia (Dunning and Förch, 1998) with the highest catch rates at depths of 350 and 500m and in Hawaiian waters from depths of about 200-700 m during the day and 0 to 400 m at night (Harman and Young, 1985). During the day it is seen on or near the ocean floor (a video can be seen here of squid in Hawaiian waters at a depth of about 400m ). This squid, in Hawaiian waters, is rarely caught on squid jigs which suggests that they are also demersal at night. In other locations, N. hawaiiensis appears to have been captured mostly by demersal trawls (Dunning and Förch, 1998) and Dunning (1998) also concluded that this species demersal day and night.
Geographical distribution
N. hawaiiensis is known from scattered localities near landmasses in the Indian and Pacific Oceans (Dunning and Försch, 1988; see map). Near Hawaii, Bower, et al. (1999) found paralarvae of N. hawaiiensis to exhibit increased abundance near islands.
References
Berry, S.S. 1918. Report on the Cephalopoda Obtained by the F.I.S. "Endeavour" in the Great Australian Bight and Other Southern Australian Localities. Biological Results of the Fishing Experiments carried on by the F.I.S. "Endeavour", 1909-1914, 4(5):201-298.
Bower, J. R., M. P. Seki, R. E. Young, K. A. Bigelow, J. Hirota and P. Flament. 1999. Cephalopod paralarvae assemblages in Hawaiian Islands waters. Marine Ecology Progress Series, 185:203-212.
Dunning, M. C. 1998. Zoogeography of arrow squids (Cephalopoda: Ommastrephidae) in the Coral and Tasman Seas, Southwest Pacific. Smithsonian Contributions to Zoology, No. 586: 435-453.
Dunning, M. C. and E. C. Förch. 1998. A review of the systematics, istribution, and biology of arrow squids of the genus Nototodarus Pfefer, 1912 (Cephalopoda: Ommastrephidae). Smithsonian Contributions to Zoology, No. 586: 393-404.
Harman, R. F. and R. E. Young. 1985. The larvae of ommastrephid squids (Cephalopoda, Teuthoidea) from Hawaiian waters. Vie Milieu, 35: 211-222.
Voss, G. L. 1963. Cephalopoda of the Philippine Islands. Bull. U. S. Nat. Mus., 234: 1-180.
Young, R. E. (1995). Aspects of the natural history of pelagic cephalopods of the Hawaiian mesopelagic-boundary region. Pacific Science, 49: 143-155.
About This Page
Richard E. Young

University of Hawaii, Honolulu, HI, USA
Michael Vecchione

National Museum of Natural History, Washington, D. C. , USA
Correspondence regarding this page should be directed to Richard E. Young at
dickphyllisyoung@gmail.com
and Michael Vecchione at
vecchioneM@si.edu
Page copyright © 2017 Richard E. Young and Michael Vecchione
 Page: Tree of Life
Nototodarus hawaiiensis .
Authored by
Richard E. Young and Michael Vecchione.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Nototodarus hawaiiensis .
Authored by
Richard E. Young and Michael Vecchione.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 29 November 2009
- Content changed 02 May 2017
Citing this page:
Young, Richard E. and Michael Vecchione. 2017. Nototodarus hawaiiensis . Version 02 May 2017 (under construction). http://tolweb.org/Nototodarus_hawaiiensis/77450/2017.05.02 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site