INTRODUCTION
The study of the evolution of the human species has been characterized by astounding discoveries, immense public interest and a copious amount of controversy. From the earliest parts of the 20th century, new fossil hominid finds have disrupted the widely held views on the evolution of our species: Ramapithecus, Raymond Dart’s ‘Taung Child’ and the infamous Piltdown Man, to name but a few. However, the history of paleoanthropological discourse, while interesting, is beyond the scope of this investigation (but see Bowman-Kruhm 2005; Cartmill et al. 1986; Delisle 2007; Lewin 1988; McCown and Kennedy 1972; Ward 2003).
The purpose of this investigation is to address the morphology, taxonomy and evolution of one particular group of hominids, the australopithecines. This diverse group of hominids first appeared in Africa sometime around 4.2 Mya (millions years ago) and diversified into a number of forms before the last recorded member of the group went extinct around 1.4 Mya. A number of species have been recovered since 1925, and will be considered here: Australopithecus anamensis, A. afarensis, A. africanus, A. garhi, Paranthropus aethiopicus, P. boisei and P. robustus. Although some classify Homo habilis as an australopithecine (e.g. Boyd and Silk, 2003), this is not the view taken in this investigation, as the consensus in the literature describes this species within the genus Homo. Figure 1 summarizes the temporal distribution of the species discussed. It should be mentioned that although many have advocated a separate genus (Paranthropus) for the three ‘robust’ australopithecines (e.g. Susman 1988), they are still discussed here with the ‘gracile’ australopithecines. It is still common to encounter texts in which these robust species are not assigned to a separate genus (e.g. Hlusko 2004; White 2002). The reasons for this will be discussed in more detail below.

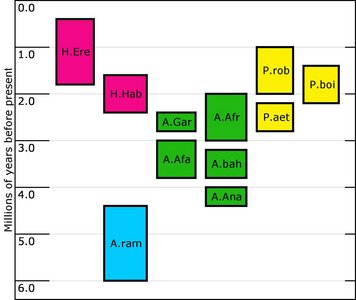
FIGURE 1. Temporal distribution of some of the species discussed in this investigation. H.ere=Homo erectus; H.Hab=Homo habilis; A.ram=Ardipithecus ramidus; A.gar=Australopithecus garhi; A.afa=Australopithecus afarensis; A.afr=Australopithecus africanus; A.bah=Australopithecus bahrelghazali; A.ana=Australopithecus anamensis; P.aet=Paranthropus aethiopicus; P. boi=Paranthropus boisei; P.rob=Paranthropus boisei
If one wishes to understand the evolution of Homo sapiens and the evolution of the genus Homo in a broader sense, it is critical to address the australopithecines. While the evolution of Homo is in itself intrinsically interesting, particularly when one considers the recent finds in the Republic of Georgia (Balter and Gibbons 2000; Gabunia et al. 2000; Gabunia et al. 2001; Rightmire et al. 2006; Schwartz et al. 2000), it will not be discussed in any explicit terms here. Instead, this investigation will deal exclusively with the evolutionary history of the australopithecines: their anatomy, diet, ecology and behavior. This will be accomplished through an in-depth examination of fossil, primatological and paleoecological evidence.
DEFINING FEATURES OF THE AUSTRALOPITHECINES
The australopithecines are somewhat hard to define as a group. Raymond Dart (1925) was the first to describe an australopithecine in the literature when he published a description of the now famous Taung Child discovered in South Africa the previous year. This individual was a juvenile specimen attributed to the species Australopithecus africanus. The name Australopithecus translates to ‘southern ape’. This nomenclature can largely be attributed to the ape-like features of the cranium and the location of its discovery (a Limestone cave in South Africa). However, since this time, the genus has been discovered in the eastern part of Africa as well. The defining features of the australopithecines as a clade can be summarized as follows (White 2002). It should be noted that these defining characteristics apply to both the ‘gracile’ (Australopithecus) and ‘robust’ (Paranthropus) varieties.
- Anatomy adapted to bipedal locomotion
- High brachial index (forearm/upper arm ratio) relative to other hominids
- Sexually dimorphic to a degree greater than Homo and Pan, but less than Gorilla or Pongo
- Height: 1.2 m – 1.5 m; Mass: 30 kg – 55 kg (estimated)
- Cranial capacity: 350 cc – 600 cc
- Postcanine dentition relatively large, enamel thickened compared to contemporary apes and humans
- Incisors and canine relatively small, little sexual dimorphism in canines compared to modern apes
The adaptation to bipedal locomotion (1) is of particular significance in human evolution. All australopithecines possess anatomical characteristics of the pelvis, femur and spinal column that facilitate bipedal locomotion. Whether or not the australopithecines were fully adapted bipeds is still hotly debated in the literature. There are several important adaptations to bipedal locomotion that can be observed on skeletal material. First, the foramen magnum is shifted forward, underneath the skull. This positioning is indicative of the angle at which the spinal chord enters the skull (Tobias, 1998). The 's' shape of the spine aids in balancing while walking on two legs (Johanson and Edgar, 2006). Adaptations more directly related to bipedal locomotion can be observed in the pelvis, the bones of the leg and the foot. The pelvis of a bipedal hominid is wide, with relatively short ilia that form a basin to support the internal organs. This arrangement facilitates the positioning of the hip muscles laterally with respect to the legs, ehancing balance while walking on two legs (Johanson and Edgar, 2006). The neck of the femur is lengthened in bipeds, adding leverage to the hip abductors and increasing the efficiency of bipedal locomotion (Boyd and Silk, 2003). Additionally, the articulation between the femur and the pelvis, and the arrangement of the knee ensure good distribution of weight while walking (Johanson and Edgar, 2006). The tibia also displays several features which indicate weight transfer from one leg to another: a relatively large proximal condyle and a near right angle between the proximal shaft and proximal articular surfaces (Klein 1999).
The first bipeds were likely hominids that predated the australopithecines. Although postcranial evidence of hominids pre-dating the australopithecines is relatively rare, the positioning of the foramen magnum on the cranium of some fossil specimens is suggestive of their status as bipeds (Ahern, 2005). This is true of both Ardipithecus ramidus (ca. 4.4 Mya) from Aramis, Ethiopia and the recently discovered Sahelanthropus tchadensis (ca. 6-7 Mya) from central Africa (Chad). Furthermore, while it has been suggested that another pre-australopithecine species (Orrorin tugenesis) was an adept biped based on its relatively complete postcranial remains (Pickford et al., 2002); others have argued that this interpretation is incorrect and suggest that Ardipithecus is ancestral to all species of australopithecines and the first true biped (Haile-Selassie, 2001). The past fifteen years have seen several important pre-australopithecine fossils emerge as potential ancestors, but the origin of the australopithecines and bipedalism remains unclear (for a more complete assessment of the origins of bipedalism, see Harcourt-Smith and Aiello, 2004; Stanford, 2003).
The high forearm/upper arm ratio (2) of the australopithecines is suggestive of their locomotion. Limb indexes such as the brachial index are useful for reconstructing the locomotor habits of hominids. Generally, primate species that are leapers have long hindlimbs (and thus low intermembral indices), suspensory species (e. g. Pongo) have high brachial and intermembral indices and quadrupedal species (Pan) have intermediate indices (Fleagle, 1997). As was already discussed, morphological evidence indicates that the australopithecines were bidepal. The fact that their brachial indices are high suggests that they may still have utilized arboreal habitats, possibly for sleeping, foraging or evading predators; these behaviors have been discussed at length by several authors (e.g. Sabater Pi et al., 1997; Sept, 1992; Sussman et al., 1984).
The degree of sexual dimorphism (3) present in the australopithecines has been hotly debated. For some skeletal samples, there is debate about whether a high degree of sexual dimorphism exists or whether there are two species of hominid present in the sample, one large and one small (Johanson and Edey, 1981). While it is by no means conclusive due to the uncertainty inherent in estimating body size from fossil specimens, it now appears that the australopithecines exhibited a degree of sexual dimorphism considerably greater than that observed in modern humans and chimpanzees, but less than that observed in gorillas (Larsen, 2003). Interestingly though, most australopithecines are relatively rare among anthropoids in being highly dimorphic in terms of body size, but not in canine size (7). The degree of sexual dimorphism is significant because this has implications for social organization and mating systems. Generally, primate species that exhibit high degrees of sexual dimorphism are characterized by intense male-male competition and a polygynous social organization (Fleagle, 1997; Plavcan and van Schaik, 1997). Unfortunately, it is difficult to use extant primates as analogies for the australopithecines because no species exhibit the same pattern of canine and body size dimorphism. However, it has been suggested that social organization was centered around multi-male co-operating kin groups (Larsen, 2003).
As was already mentioned, body size is notoriously difficult to estimate from fragmentary fossil specimens. Furthermore, some species of australopithecine are known from a very small set of remains, making it even more difficult to estimate body size. However, several species are represented by a relatively good record and estimates of their body size and stature (4) are presented below in Table 1. For comparitive purposes, the sizes of several extant species are included (from Ankel-Simons, 2000; Johanson and Edgar, 2006; Jones et al., 1996; Jungers, 1988). This table demonstrates a relatively uniform distribution in body size across the australopithecines. In terms of body mass, they are relatively comparable to Pan, but are considerably taller, likely as a result of their adaptation to bipedal locomotion.

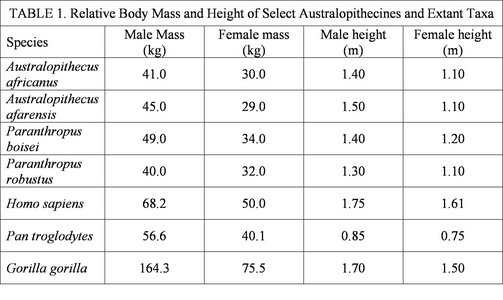
Table 1 demonstrates the relative body sizes of the australopithecines compared to several extant taxa. Furthermore, the degree of body size sexual dimorphism can also be observed.
Although a general trend in human evolution is an increase in cranial capacity, this trend does not really become marked until the appearance of the genus Homo. The cranial capacities of the australopithecines (5) are relatively similar (see Table 2). It should be pointed out that these cranial volumes be approached with caution and considered only with respect to overall body mass. The cognitive abilities of the australopithecines are largely unknown, but clear evidence indicates that at least some species were manufacturing and using crude stone tools by 2.6 million years before present (Semaw et al., 2003). It is likely that tools of a more perishable variety (composed of organic materials) were made and used at an even earlier date, but taphonomic processes do not allow for the preservation of such materials (Schick and Toth, 1993). There is no indication that the australopithecines possessed any linguistic capabilities. The implications of the cognitive abilities of the australopithecines will be addressed in more detail below.

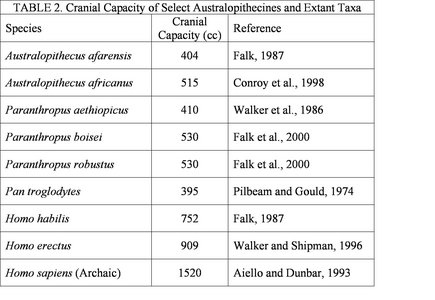
Table 2 details the average cranial capacities of several australopithecine species and extant primate taxa. It should noted that these estimates are often based on a very small number of specimens.
The dentition of the australopithecines is particularly important because the most abundant fossils are isolated teeth, and an examination of the morphology of teeth can be used to determine both phylogenetic relationships, diet and social organization. The postcanine dentition is quite large and the enamel is very thick (6) in the australopithecines, especially Paranthropus. Extant primate species with thick enamel tend to feed on hard objects, such as fibrous plant material, nuts and seeds; a similar diet has been proposed for the australopithecines based on their dentition (Teaford, 2007).
THE AUSTRALOPITHECINE DIET
Reconstruction of paleodiet has been an extremely prevalent aspect of human origins research since the beginning. Views on what the earliest ancestors of humans may have been eating have changed considerably in the last fifty years, and there is still no consensus on the part of paleoanthropologists.
Early paleoanthropological discourse was centered heavily on the consumption of meat in the early hominid diet (see Krantz, 1968; Lee and DeVore, 1968; Washburn, 1957). A particularly violent image was constructed for the australopithecines, often involving intense conflict (see Keyes Roper, 1969), cannibalism (e.g. Emiliani, 1968) and hunting of game (e.g. Brown and Lahren, 1973; Isaac, 1983; Krantz, 1968). Interestingly, these hypotheses were based on little evidence and were eventually abandoned. One of the major implications of meat-eating was an associated increase in cranial capacity (Aiello and Wheeler, 1995), but this trend was not observed until the appearance of the genus Homo.
While meat-consumption remained an important point of discussion throughout the twentieth century, there was an increase in the focus on the plant component of early hominid diets beginning in the late 1970s and early 1980s (e.g. Dahlberg, 1981; Tanner, 1981). The shift in focus was co-incident with an increase in the amount of discussion of the role of women in human evolution. The contemporary literature considers both the consumption of meat and plant foods.
The context in which meat was thought to be consumed has changed dramatically. There has been an increase in the consideration of the scavenging rather than hunting capabilities of early hominids (e. g. Blumenschine, 1987). The scavenging hypothesis is largely constructed on the interpretations of cut marks on mammalian remains and reconstructions of paleoecological conditions, carnivore guild for example (Shipman, 1986). The accumulation of bones at some sites has been used to suggest the presence of ‘home bases’ for early hominids (Potts, 1984). However, more recent interpretations of these faunal remains indicates that it is very questionable whether early hominids were actually responsible for the accumulation of these bones (Rose and Marshall, 1996).
There has also been a shift in opinion with respect to the potential hunting abilities of early hominids, with a move towards viewing them as being incapable of competing with larger carnivores due to their limited cognitive abilities, crude toolkit and relatively small size (e.g. Potts, 1984). However, given the amplitude of evidence for hunting behavior in Pan that has emerged over the past forty years (Stanford, 1996, 1999; Takahata et al., 1984; Teleki 1973; Wrangham and van Zinnicq Bergmann-Riss, 1990), it is unreasonable to suggest that early hominids were incapable of hunting behavior. Furthermore, an analysis of the physiology of the gastrointestinal tract and scavenging behavior in contemporary chimpanzees and baboons reveals a staunch avoidance of carrion due to the increased risk of internal parasites (Ragir et al., 2000).
Scavenging does become a plausible means of subsistence when cooking food is an option (Ragir et al., 2000), but there is no reason to believe that early hominids were able to control fire for cooking (Klein, 1999). Based on these data, it is reasonable to suggest that some species of early hominid were hunting and consuming meat from small mammals, reptiles and birds, in a way similar to modern chimpanzees. However, stronger zoo-archaeological evidence for this behavior must be discovered before anything conclusive can be said about meat-consumption in early hominids.
Much clearer evidence has emerged with respect to the plant component of australopith diets. Stable isotope analyses have revealed that the australopithecines consumed a diet that was rich in C4 plants (grasses, sedges), or animals that consumed C4 plants (Sponheimer et al., 2005; van der Merwe et al., 1999, 2003). An amalgamation of the carbon isotope data reveals that Australopithecus and Paranthropus were both distinct from contemporaneous C3 consumers (browsing/frugivorous bovids and giraffids) and C4 consumers (grazing bovids and equids), but very similar to one another (Sponheimer et al., 2007). While these data are intriguing, they are not able to directly address exactly which C4 resources were consumed by the australopithecines.
Based on the preceding discussion of meat-consumption in early hominids, it is unlikely that the diet was largely meat-based, although C4 consuming animals could have contributed somewhat to the stable isotope values obtained. It is much more likely that the carbon isotope data reflects plant resources consumed by early hominids. Some modern species of primate, several species of baboons in particular, also consume diets rich in C4 plants, specifically grasses (Sponheimer et al., 2007). This type of diet, however, is relatively nutrient poor compared to the frugivorous diets of many modern ape species. The possibility that australopithecines heavily exploited underground storage organs (USOs) has been discussed recently in the literature (e. g. Laden and Wrangham, 2005). These USOs are relatively rich in nutrients and could, in part, account for the C4 component of early hominid diets.
Although Paranthropus and Australopithecus exhibit similar carbon isotope values, it is unlikely that their diets were the same. The robust australopithecines possessed greatly enlarged cheek teeth with very thick enamel when compared with the ‘gracile’ australopithecines (See Figure 2). Grine and Martin (1988) suggest that this increased enamel thickness was related directly to some functional dietary adaptation. Furthermore, the mandible in Paranthropus was much more robust and the temporalis and masseter muscles were considerably larger (Fleagle, 1997). Taken together, this indicates that Paranthropus was likely consuming some type of very hard material, possibly nuts and/or seeds. These food items could potentially have come from C4 plants, so this hypothesis does not negate the stable isotope data.

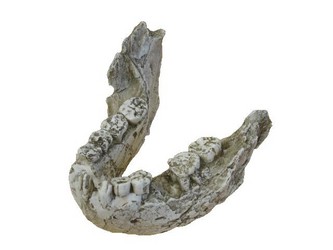
FIGURE 2. Mandible of Paranthropus boisei (KNM-ER 729). This specimen demonstrates the immense size of the mandible and massive cheek teeth (premolars and molars) in Paranthropus. © 2007 Paul Szpak
The data obtained thus far are inconclusive with respect to paleodiet. However, based on the evidence a mixed-mode of subsistence could be proposed for early hominids, with a high reliance on underground storage organs, complemented by other plant materials (seeds, nuts, fruits) and a small component of hunted game (small mammals, reptiles and birds). Paranthropus probably consumed more fibrous plant materials based on its dental morphological characteristics. For those interested in a more extensive examination of early hominid diet, consult the volume edited by Ungar (2007).
CLIMATE AND ECOLOGY
The reconstruction of past climatic and ecological conditions is of utmost importance for addressing the evolution of early hominids. Morphological analyses of skeletal remains become much more significant when they are placed within a broader environmental and ecological context.
The Late Pleistocene (3.0-2.0 Mya), a period of particular significance for the evolution of the australopithecines, was characterized by marked climatic and ecological changes in East Africa. Terrestrial paleoclimatic indices indicate that the climate changed from warm and wet to a more seasonal climate, which was cool and dry (deMenocal and Bloemendal, 1995). This general trend began some time between 3.5 and 3.2 Mya and became more pronounced around 2.6 Mya (Dupont and Leroy, 1995); maximum aridity was reached 1.8 Mya (Bonnefille, 1995). Analyses of sedimentation deposited in caves support these interpretations (Partridge et al., 1995).
Paleoclimatological data related to earlier periods (>3.5 Mya) are less well known. Thus, it is difficult to determine exactly what ecological conditions were like when the australopithecines emerged in Africa. More extensive paleoecological analyses must be carried out with respect to this time period in order to better understand the origins of the australopithecines as a group.
Pollen analyses have been used fairly extensively to reconstruct the environmental context of early hominid sites (e. g. Carrión and Scott, 1999; Domìnguez-Rodrigo et al., 2001; Zavada and Cadman, 1993). An examination of the data obtained from pollen analyses reveals that there is no clear way to characterize the environment of early hominid sites. A high degree of variability exists with respect to ecological conditions at these sites. Plant ecosystems at early hominid sites range from subdesertic steppe to Afro-alpine heath moorland, but do not show any evidence of closed rainforest (Bonnefille, 1995). This diversity in ecology and plant resources fits well with the generalized mixed-mode of subsistence presented earlier (utilization of a wide variety of plant and animal resources) for these early hominids. For those interested in exploring paleoclimate and paleoecology further, consult the volume edited by Vrba et al. (1995).
AUSTRALOPITHECINE TECHNOLOGY
The technological capabilities of the austrlaopithecines have been widely discussed in the literature, with much disagreement. Early studies of tool-manufacturing and tool-using capabilities of the early hominids were quite problematic. In southern Africa, Dart (1960) attributed a toolkit based on bone, teeth and horn to the australopithecines. However, the proposed ‘osteodontokeratic culture’ of the australopithecines was not particularly well received among most paleoanthropologists and failed to gain acceptance (see Wolberg, 1970). In east Africa, the earliest documented stone tools of the Oldowan Industry, were initially attributed to Paranthropus boisei; however, this changed with the discovery of the more advanced Homo habilis, who was then heralded as the first stone tool maker in the hominid lineage (Tobias, 1965). As more lithic evidence became available, this scenario quickly became untenable.
The earliest document evidence of stone tool technology occurs at ~2.6 Mya in Ethiopia (Semaw et al., 2003). These and all other lithic assemblages until ~1.7 Mya are known as the Oldowan Industry, named after their site of initial discovery, Olduvai Gorge. The Oldowan Industry is distributed throughout Africa and has recently been discovered in Europe in association with the recently discovered hominid remains in the Republic of Georgia (Gabunia et al., 2001). The nature of the tools themselves is highly debatable. It was initially purported that the technology was based around large cores that were used as general purpose tools (choppers) and flakes (removed from the cores) that may have also been utilized as scrapers, burins or knives (see Leakey, 1971). Both the flakes and cores are very simple in nature, involving minimal reduction to reach their final stage. If one is not well-versed in early lithic technology, it is very easy to view these tools (especially the cores) as nothing more than rocks. For those wishing to see what these tools looked like, Leakey (1971) and Schick and Toth (1993) present very good illustrations of the Oldowan Industry. The cores were seen as the dominant tool-type of the Oldowan for many years.
More recently, it has been suggested that the cores were really nothing more than byproducts of flake manufacture. According to this view, it was the flakes that were the desired tool (Toth, 1985). This is in much better accordance with what is known about the cognitive abilities of the australopithecines. The manufacture of a core tool involves a mental template for what that tool should look like and implies a degree of intelligence, which may have been beyond the australopithecines (Toth, 1985). Conversely, flakes that are struck from a core and utilized require considerably less in terms of mental capabilities, and this is perhaps comparable to the amount of foresight required for a chimpanzee to select an appropriate branch for termite fishing or rock for nut-cracking. The earliest Oldowan tools do not show any evidence of re-touching (Semaw, 2000) and they were constructed of locally abundant materials (Toth, 1985), indicating that hominids were not particularly selective in terms of raw materials. This fits with an interpretation of little mental foresight in the early Oldowan toolmakers.
Because of taphonomic processes, lithic technology is all that preserves from the Pliocene and Pleistocene. However, this does not suggest that australopithecine technology consisted only of simple stone tools. Rather, it is very likely that the australopithecines utilized a wide variety of tools made of perishable materials (such as digging sticks and probes), what have been described as instruments by Oswalt (1976). These instruments are found in all toolkits of modern hunter-gatherer groups (Torrence 2001), and the use of these tools has been well documented in Pan troglodytes (McGrew, 1992). Given the universality of these types of tools and the notion that Pan troglodytes is the best analogy for early hominid behavior (McGrew, 1993), the australopithecines likely used wooden instruments fairly extensively, possibly for digging up USOs or immobilizing small animal prey.
Determining exactly what the earliest tools were used for is a difficult task to say the least. A number of potential uses have been proposed, including: butchery, woodworking, hide scraping, hide slitting and grass cutting (Schick and Toth, 1993). However, butchery is the only one of these that leaves any visible evidence in the archaeological record. Nelson (1991) suggests that the items in a toolkit with few tool types (the Oldowan would be a good example of this) be versatile and flexible. In other words, the few tool types that are present would be used in a wide variety of contexts. In this way, it is sensible to suggest that the australopithecines were utilizing these simple (but versatile) flake tools for a wide variety of tasks, including, but not limited to those mentioned above.
It is notoriously difficult to directly correlate a stone tool technology with any particular species of hominid. However, given the temporal depth of the Oldowan tools (~2.6 Mya), it is clear that at least some species of australopithecine were making these tools, as Homo did not appear for several hundred thousand years. Some have suggested that only Paranthropus made and used stone tools, and Australopithecus did not (Fleagle, 1997). This seems unlikely though, given the distribution of stone tool bearing sites and the fact that many of them are not found in association with Paranthropus. However, this is not to suggest that Paranthropus was not capable of manufacturing stone tools. Until more sites are found and better associations can be made between specific species of early hominid and lithic assemblages, it is unclear which species manufactured stone tools and which did not. Any and all species of australopithecine inhabiting the African landscape between 2.6 Mya and 1.7 Mya could be responsible for these tools.
Documenting the first evidence of stone tool manufacture establishes this behaviors’ antiquity in the hominid lineage, but it does not say anything about why this behavior may have occurred. The most widely discussed hypothesis involves climatic change and a shift in resource availability around 2.6 Mya. As was already discussed, a general trend towards a more seasonal and dry environment became pronounced at ~2.6 Mya. According to this hypothesis, sometimes referred to as the ‘turnover pulse hypothesis’ (Vrba, 1985), when presented with more seasonal environments, early hominids adopted stone tool use to cope with a more variable resource base. While this hypothesis has been relatively well-received (see Plummer, 2004), it remains difficult to test with empirical archaeological evidence. There may be other extenuating factors which contributed to the development of the first stone tools.
For those interested in more in-depth considerations of the earliest stone tools, there is a substantial amount of literature published on the subject. Schick and Toth’s (1993) work remains a classic and a must-read for anybody interested in the origins of stone tools. More recently, Plummer (2004) provides a very good review of the development of the Oldowan.
OVERVIEW OF AUSTRALOPITHECINE SPECIES

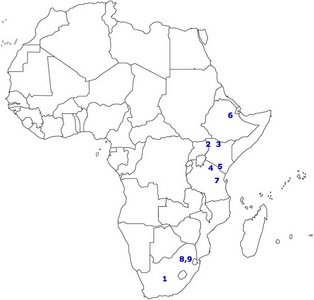
FIGURE 3. Map of Africa showing the distribution of several important sites.
1) Taung, 2) West Turkana, 3) East Turkana, 4) Olduvai Gorge, 5) Peninj, 6) Hadar, 7) Laetoli, 8) Sterkfontein, 9) Swartkrans. © 2007 Paul Szpak
Figure 3 shows the spatial distribution of some of the most significant australopithecine sites. Generally, the australopithecines can be divided into two broad categories based on their dental characteristics: the ‘gracile’ (Australopithecus) and robust (Paranthropus) australoptihecines. The traits that are used to differentiate these genera are summarized in Table 3 (compiled from Fleagle, 1997). The following section will examine each species of australopithecine individually, focusing largely on the morphological characteristics of each species.

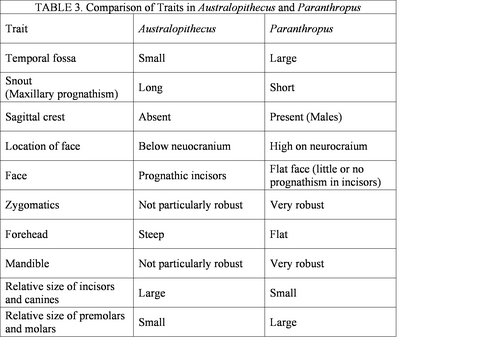
Table 3 shows some of the morphological characteristics that can be used to dinstinguish Australopithecus from Paranthropus. © 2007 Paul Szpak
Australopithecus anamensis
- Type Specimen: KNM-KP 29281 [Mandible] (Leakey et al., 1995)
- Location: East Africa (Kenya, Ethiopia)
- Age: 4.17-3.9 Mya
- Cranial Capacity: Unknown
- Estimated Size: 47 kg – 55 kg
Australopithecus anamensis (anem is the Turkana word for lake) is the earliest and one of the most poorly known members of the genus Australopithecus. This species is largely known from teeth and fragmentary pieces of maxillae and mandibles; very limited postcranial material has been recovered (Klein, 1999). However, important among these limited postcranial remains is the proximal end of a tibia, which demonstrates morphological characteristics that demonstrate A. anamensis was a habitual biped (Leakey et al., 1995). However, the humerus and radius of A. anamensis show characteristics that suggest it was also adept at moving through the trees (Klein, 1999). Additionally, it has been suggested that, based on the limited postcranial material available, A. anamensis exhibited a considerable degree of body size sexual dimorphism (White, 2002).
The mandibular morphology of A. anamensis is quite distinct. The postcanine tooth rows are arranged essentially parallel to one another. This condition is present in Pan, but not in other species of hominid, who tend to have a much more parabolic arch in their dentition. Furthermore, the canines of A. anamensis are considerably larger than any other species of hominid (Leakey et al., 1995). Again, this condition is similar to that observed in Pan, but rare among the Hominidae.
There are, however, several important traits that A. anamensis shares with other australopithecines, particularly A. afarensis. The enamel is quite thick, and the molars are large and broad (Klein, 1999). Additional similarities to A. afarensis (particularly the earliest specimens) in the morphology of the mandible, maxilla and postcranial skeleton are also present, which may indicate a close evolutionary relationship between the two species (Klein, 1999). However, given the lack of well-preserved A. anamensis specimens, nothing definitive can be said at this point.
Australopithecus afarensis
- Type Specimen: L.H. 4 [Mandible] (Johanson et al., 1978)
- Location: East Africa (Kenya, Ethiopia, Tanzania, Chad)
- Age: 3.6-2.9 Mya
- Cranial Capacity: 350 cc – 600 cc
- Estimated Size: 30 kg – 55 kg


FIGURE 4. Distal end of the femur and proximal end of the tibia of Australopithecus afarensis (A.L. 129-1). The articulation between these two bones is almost identical to that of modern humans. © 2007 Paul Szpak
Australopithecus afarensis (afarensis derives from the Afar, a pastoralist group living in Ethiopia) is perhaps the most well-known member of the genus. This is largely due to the very famous and relatively complete female specimen (A.L. 288-1) discovered in the 1970s known informally as ‘Lucy’. This species is well-represented in the fossil record (especially compared to A. anamensis) with plentiful cranial and post-cranial remains. Thus, much more is known about its anatomy and behavior than earlier hominids.
Although it was initially believed that A. afarensis represented the first case of habitual bipedalism in the hominid fossil record (Johanson and Edey, 1981), it is now clear that this is not the case, as was discussed previously. However, this does not suggest that this species of hominid is in any way less significant.
The cranium of A. afarensis possesses many apelike characteristics. The cranial morphology of this species is quite well known, based largely on three nearly complete specimens: A.L. 333-105, A.L. 444-2 and A.L. 822-1 (for an extremely detailed examination of A. afarensis cranial morphology, see Kimbel et al., 2004). The cranial capacity varies relatively widely in this species; this can be attributed to a high degree of sexual dimorphism. Initially, the range of cranial capacity in A. afarensis was reported to be 380 cc – 485 cc (Klein, 1999), but the relatively recent discovery of the male A.L. 444-2 skull has increased the high end of this spectrum considerably (Kimbel et al., 2004). This specimen had a particularly robust mandible, well-developed bony areas for attachment of the temporalis and masseter muscles and relatively large canine teeth (Johanson and Edgar, 2006). Female specimens (e.g. A.L. 417-1) are considerably smaller, have much smaller canines and less pronounced areas for muscle attachment (Rak et al., 1996). This degree of sexual dimorphism is considerably greater than that observed in more recent hominids and modern humans, and is similar to the degree expressed in some extant great ape species.
Although the brain of A. afarensis was relatively small compared to other hominids, endocasts reveal that A. afarensis’ brain may have undergone a substantial reorganization. The parietal cerebral cortex is markedly enlarged and exhibits a different type of organization than observed in apes, characteristics firmly associated with more recent hominids and modern humans (Holloway, 1983). Due to the lack of complete cranial remains of earlier hominids it is presently unknown whether A. afarensis was the first hominid to undergo a substantial reorganization of this part of the brain.
The face of A. afarensis projects strongly forward below the nose, thus it can be said to exhibit a strong degree of what Rak (1983) refers to as alveolar or sub-nasal prognathism. This contrasts sharply with most other australopithecines and especially with members of the genus Homo, all of whom possess relatively flat faces with considerably less sub-nasal prognathism.
The canines are quite large in this species and are considerably larger in males than females. This, combined with the high degree of body size sexual dimorphism may have important implications for social organization in this species, possibly reflecting intense male-male competition and a polygynous mating system, as was discussed previously.
A. afarnesis possesses a diastema (space between the upper second incisor and canine). This gap occurs due to the large size of the lower canine and is observed in modern great ape species, but not in other hominids (Klein, 1999). Additionally, the tooth rows on the maxilla converge posteriorly (Kimbel et al., 2004), making the maxilla appear as though it were pinched at the posterior end.
A recently discovered partial mandible from Chad is very similar to other specimens of A. afarensis, but has been assigned by some (Brunet et al., 1996) to a separate species, Australopithecus bahrelghazali based on some differences in tooth morphology. However, additional fossil material must be recovered and examined before this specimen is designated as a separate species. Some have suggested that this specimen be lumped with A. afarensis for the time being (Klein, 1999) and this is the view taken in this investigation.
Based on the wear patterns observed on the teeth of several A. afarensis specimens, it has been suggested that their diet was largely folivorous and was most similar to modern gorillas (Johanson and Edgar, 2006). This suggestion seems reasonable. Stone tool use has not been documented at the time during which A. afarensis lived. However, it is entirely possible that this species manufactured expedient tools of wood, grass or other organic materials in a fashion similar to modern chimpanzees; but, this cannot be demonstrated directly in the fossil record.
The postcranial skeleton of A. afarensis exhibits a mix of apelike and humanlike features. The femur is relatively short, the forearms appear to have been relatively long and very powerful, the phalanges of the hands and feet were curved and the morphology of the glenoid fossa (on the scapula) appears to have been more similar to modern chimpanzees (Klein, 1999). All of these anatomical features indicate that A. afarensis was adept at climbing trees, potentially for foraging or sleeping.
While A. afarensis may have been able to climb trees with relative ease, it was also fully adapted to bipedal locomotion. Much of what is known about the postcranial anatomy of A. afarensis has been obtained from analyzing the famous skeleton ‘Lucy’. Several important anatomical characteristics demonstrate the bipedality of A. afarensis, including: a forwardly positioned foramen magnum, a very humanlike foot with an arch and non-opposable big toe, the angle between the proximal end of the tibia and distal end of the femur is essentially the same as in modern humans (see Figure 4) and the pelvis is short, broad (basin-shaped) and has a posteriorly extended ilium (summarized from Johanson and Edey, 1981; Klein, 1999). All of these traits facilitate bipedal locomotion and are observed in modern humans, but not in the great apes.
While A. afarensis may not be the first biped, it is the oldest fossil hominid to demonstrate evidence of all of the characteristics discussed above. Other, older, hominids such as Ardipithecus ramidus possess some, but not all of these key bipedal features. This is largely due to the relative completeness of the fossil record with respect to A. afarensis. The evolutionary significance of these traits will be considered below.
Australopithecus africanus
- Type Specimen: Taung Child [Complete Skull] (Dart, 1925)
- Location: Southern Africa (South Africa)
- Age: 3.0-2.0 Mya
- Cranial Capacity: 430 cc – 520 cc
- Estimated Size: 40 kg – 50 kg


FIGURE 5. One of the most famous examples of Australopithecus africanus, known as Mrs. Ples (STS 5). This specimen is a female. Note the relatively high degree of sub-nasal prognathism (although not as great as is observed in A. afarensis or P. aethiopicus). © 2007 Paul Szpak
Australopithecus africanus (southern ape-man of Africa) was the first australopithecine to be described. This species is one of two exclusively South Africa species of australopithecine (the other being Paranthropus robustus). This presents problems for dating because South African sites tend to be located in limestone caves which are not conducive to the absolute dating methods employed in the more stratigraphically well-document sites of East Africa. As such, the dates associated with most South African specimens should be treated with caution because they are derived largely from relative methods (dating associated faunal remains for example).
There are several relatively well-known specimens of A. africanus, including the Taung Child (Dart, 1925), STS 5 (see Fig. 5), also known as Mrs. Ples (Broom, 1947) and STS 71. As with most hominid species, the majority of the fossil material consists of cranial, maxillary, mandibular and dental elements, but a significant amount of postcranial material has also been recovered for A. africanus (e.g. STS 14). These postcranial remains demonstrated a similar suite of characteristics observed in A. afarensis, including the characteristic angle between the femur and tibia and the broad pelvis with a posteriorly oriented ilium (Larsen et al., 1998). However, there were several important postcranial differences between A. afarensis and A. africanus. First, the big toe of A. africanus appears to be considerably divergent from the rest of the foot, although not completely opposable as is observed in great apes (Clarke and Tobias, 1995). This contrasts sharply with A. afarensis whose big toe was not at all divergent. Thus, it is possible that an evolutionary reversal took place in A. africanus. Like A. afarensis, A. africanus had a relatively large forearm, indicating an adaptation to tree-climbing (McHenry and Berger, 1998). However, the forearm was even larger relative to the leg and upper arm in A. africanus which might indicate an even stronger connection to life in the trees (Klein, 1999).
The cranial capacity of A. africanus was similar to that of A. afarensis, but the overall craniofacial morphology was quite different. A. africanus had a less prognathic face and the forehead was much more arched (Rak, 1983). Together, these traits produced a flatter face than observed in A. afarensis. Additionally, the temporal and nuchal lines on the skull did not show the heavy ridges present in A. afarensis, indicating a reorganization of the temporalis and masseter muscles (Klein, 1999). This may indicate a different dietary adaptation, with less reliance on tough folivorous vegetation requiring substantial development of the anterior dentition and more reliance on softer foods that do not require a great amount of effort from the anterior dentition such as fruits, seeds and nuts. Furthermore, the post-canine teeth were enlarged with a tendency for the premolars to be similar to the molars and the mandible was more robust (Klein, 1999). Overall, the dental morphology of A. africanus indicates an increased reliance on the post-canine dentition for food processing.
There was little sexual dimorphism in canine size in A. africanus. Additonally, the presence of a diastema is rare and when present less marked than in A. afarensis (Klein, 1999). The degree of body-size sexual dimorphism in A. africanus was slightly less than that observed in A. afarensis, but still greater than in modern humans (see Table 1). This pattern of significant body-size dimorphism, but little canine dimorphism does not occur elsewhere in primates. As such, it is difficult to make inferences with respect to the social organization of A. africanus based on these data. However, it is possible that it was markedly different from that of A. afarensis. Given the age of A. africanus, it is entirely possible that this species manufactured stone tools. However, directly associating stone tools with particular hominid specimens is notoriously difficult, especially in South Africa where accurate dating is problematic.
Australopithecus garhi
- Type Specimen: BOU-VP-12/130 [Cranium] (Asfaw et al., 1999)
- Location: East Africa (Ethiopia)
- Age: 2.5 Mya
- Cranial Capacity: 450 cc
- Estimated Size: Unknown
Australopithecus garhi (garhi translates to surprise in an African dialect) is a relatively recently discovered species of australopithecine that is known only from the type specimen (BOU-VP-12/130). Limited postcranial remains were recovered with this specimen, including a femur, humerus, radius and ulna, all of which were partial (Asfaw et al., 1999). Due to the lack of fossil evidence, relatively little is known about the anatomy and behavior of A. garhi.
The postcranial remains that have been recovered are fragmentary, but appear to be quite similar to those of A. afarensis (Asfaw et al., 1999). Based on this, it has been suggested that A. garhi was also an adept biped, although conclusive evidence of this has not been recovered to date. Furthermore, the postcranial remains indicate apelike arm/leg proportions, similar to those observed in A. afarensis (Asfawe et al., 1999).
The cranium of A. garhi is similar to A. afarensis in many ways. A relatively high degree of subnasal proganthism is present and the canines are quite large. In addition to this, the majority of the other cranial and dental features are also reminiscent of A. afarensis (White, 2002). However, there are several differences, the most important of which relates to the postcanine dentition. The premolars and molars of A. garhi are enlarged, as is the case in A. africanus and Paranthropus, but the incisors and canines are not reduced as they are in these species. Based on this, it is possible that A. garhi was exploiting a much more varied diet, which included both leaves (evidenced by the large anterior dentition) and nuts, fruits and seeds (based on the enlarged postcanine dentition). This shift in dietary adaptation may have been the main difference between A. garhi and A. afarensis, who otherwise appeared to be very similar.
The degree of body size and canine dimorphism is not clear in A. garhi, but based on its close affinity to A. afarensis in the majority of its cranial traits, it has been suggested that a similar pattern of body size dimorphism should be observed in future specimens of A. garhi (Asfaw et al., 1999). Until this is demonstrated, it is very difficult to make any assertions about the social organization of A. garhi. It is possible that A. garhi may have been one of the first, if not the first, species of hominid to manufacture stone tools based on its age. However, to this point, stone tools have not been found in direct association with the limited fossil remains of A. garhi.
Paranthropus aethiopicus
- Type Specimen: Omo-18-(1967)-18 [Mandible] (Arambourg and Coppens, 1968)
- Location: East Africa (Kenya, Ethiopia)
- Age: 2.7-2.3 Mya
- Cranial Capacity: 420 cc
- Estimated Size: Unknown


FIGURE 6. The famous 'Black Skull', also known as KNM-WT 17000. Note the long snout and high degree of alveolar proganthism. Also notice the relatively small braincase and extremely prominent sagittal crest. This specimen is a male and although the crowns of the teeth and mandible were not preserved, they would have been massive. © 2007 Paul Szpak
Paranthropus aethiopicus (species name derived from Ethiopia) is the oldest and most poorly known robust australopithecine. Several fragmentary remains have been recovered, but the most famous and diagnostic fossil specimen is KNM-WT 17000 also known as the Black Skull (see Fig. 6) due to its dark colour derived from the manganese-rich deposits where it was found (Simons, 1989). Most of what is known about P. aethiopicus is derived from this specimen.
The cranium of P. aethiopicus is very robust, possessing a small cranial capacity (~420 cc) and a large, prognathic face. The degree of sub-nasal prognathism is quite pronounced in this species and is similar to that observed in A. afarensis (Rak, 1983). P. aethiopicus possessed massive sagittal and nuchal crests for the attachment of the chewing muscles, which must have been massive. Based on the size of the sagittal crest in KNM-WT 17000, it is presumed that this specimen is a male (Walker et al., 1986). The base of the cranium in P. aethiopicus was also very similar to that of A. afarensis (Klein, 1999). Additionally, P. aethiopicus possessed a maxilla that was posteriorly convergent, another feature shared with A. afarensis. However, this species differed from A. afarensis in several important ways.
One of the most important features differentiating P. aethiopicus from A. afarensis relates to the size of the postcanine dentition. P. aethiopicus possessed extremely large molars and molarized premolars with very thick enamel and anterior dentition that was quite reduced in size. This ‘hyper-masticatory’ adaptation was characteristic of all members of the genus Paranthropus (McHenry, 1994). In addition to this, P. aethiopicus possessed a heart-shaped foramen magnum (Kimbel et al., 1988) and a markedly different morphology in the middle part of the face (Klein, 1999). These features further differentiated P. aethiopicus from A. afarensis.
Based on the immense size of the postcanine dentition, the massive chewing muscles and the thickness of the enamel, it is probable that P. aethiopicus consumed a diet largely of hard material that required extensive mastication, nuts and seeds for example. Unfortunately, because of the rarity of this species in the fossil record, adequate studies of microscopic wear patterns and/or stable isotope analyses have not been conducted thus far.
Although very little postcranial material of P. aethiopicus has been recovered, enough material has been obtained to suggest that this species was an adept biped and exhibited a high degree of sexual dimorphism. The difference in size between the Omo-18 type specimen adult mandible and the KNM-WT 170000 cranium suggests that this species exhibited considerable body size sexual dimorphism, possibly similar to that observed in A. afarensis (Klein, 1999). The postcranial remains indicate bipedal locomotion, and arm proportions that indicate at least some adaptation to life in the trees (Klein, 1999).
Paranthropus robustus
- Type Specimen: TM-1517 [Cranium] (Broom, 1938)
- Location: Southern Africa (South Africa)
- Age: 1.7 Mya
- Cranial Capacity: 530 cc
- Estimated Size: 32 kg – 40 kg


FIGURE 7. Paranthropus robustus (SK-48). Although this cranium is distorted, the sagittal crest can still be seen (although it is not as prominent as in KNM-WT 17000). This specimen also possesses very large molars and premolars. © 2007 Paul Szpak
Paranthropus robustus represents the South African version of the robust australopithecines. It shares several features with both P. boisei and P. aethiopicus, including a prominent sagittal crest in males, considerable body-size sexual dimorphism, robust zygomatics and mandible, flat face with little sub-nasal prognathism and markedly decreased anterior dentition with extremely, thick enameled and large post-canine dentition (Fleagle, 1997).
Several relatively complete and well-known cranial samples have been recovered representing P. robustus, including: SK-46 (Broom, 1949) and SK-48 (Broom and Robinson, 1950). As was already discussed, the dating of these specimens has been difficult due to their depositional contexts. The cranial capacity of P. robustus is slightly larger than P. aethiopicus, but not substantial. However, it has still been suggested that the cognitive capabilities of the more recent members of Paranthropus were considerably greater than those of P. aethiopicus and the earliest members of the genus Australopithecus (Klein, 1999). Some have even suggested that Paranthropus and not Australopithecus was responsible for generating the first stone tools (Fleagle, 1997), although this lacks supporting archaeological evidence.
The size of the post-canine dentition and the thickness of the enamel in P. robustus are considerably larger and thicker than they are in P. aethiopicus and all other species in the genus Australopithecus (Grine and Martin, 1988). Based on this, it is reasonable to suggest that P. robustus was fully adapted to a diet of tough plant material that required considerable chewing to process.
The postcranial remains that have been recovered, largely from Swartkrans in South Africa, indicates that P. robustus was a habitual biped, that it may have had less reliance on tree-climbing than earlier species (White, 2002), although additional fossil material is required before this issue can be adequately addressed.
Paranthropus boisei
- Type Specimen: O.H. 5 [Cranium] (Leakey, 1959)
- Location: East Africa (Tanzania, Kenya, Ethiopia)
- Age: 2.3-1.4 Mya
- Cranial Capacity: 530 cc
- Estimated Size: 34 kg – 49 kg

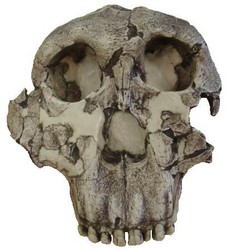
FIGURE 8. Type specimen of Paranthropus boisei (O.H. 5). Notice the flat, dish-shaped face and lack of sub-nasal prognathism that was characteristic of early members of Australopithecus and Paranthropus aethiopicus. © 2007 Paul Szpak
Paranthropus boisei, the east African version of the robust australopithecines, was also the last known australopithecine to inhabit the planet, becoming extinct ~1.4 Mya. Charles Boise was the principal financial backer of the expedition that discovered the type specimen, O.H. 5 (see Fig. 8), in 1959, and this species was named after him. Originally classified as Zinjanthropus boisei by Louis Leakey in 1959, this species was later referred to as Australopithecus boisei. It is sometimes considered an east African variant of Australopithecus robustus and now is generally referred to as Paranthropus boisei in the literature. In addition to this, P. aethiopicus was initially classified as A. boisei. Needless to say, the nomenclature for this species can be quite confusing to say the least.


FIGURE 9. Photograph of KNM-ER 406 (Paranthropus boisei). This well-preserved specimen is a male. The sagittal crest can be seen on the cranium. The zygomatics are also very robust and flared. As with O.H. 5, the face is much flatter than in earlier australopithecines. © 2007 Paul Szpak
Several important fossil specimens have been recovered representing P. boisei, including: O.H. 5 (type specimen), KNM-ER 406 (see Fig. 9) and KNM-ER 732 (Leakey, 1971; Leakey et al., 1971). In most cranial aspects, P. boisei is quite similar to P. robustus, with a few notable differences. In general terms, the robust features of the cranium of P. robustus are exaggerated in P. boisei (Klein, 1999). For example, the zygomatics are more flared and more robust, the sagittal crest is more pronounced in males and the post-canine dentition is even larger with even thicker enamel. It is because these two species seem to differ mainly in the degrees to which they express these traits that some have suggested that P. robustus and P. boisei are in fact the same species (Klein, 1999). One of the most important differences between the two species relates to, not surprisingly, their teeth. Studies of microwear indicate a marked dietary difference between P. robustus and P. boisei. The former consuming hard plant foods (grasses, leaves, nuts, seeds) and the latter focusing on fruits and tubers with little reliance on grasses and other tough plant materials (Grine, 1986; Walker, 1981). Based on this, and their geographical separation, it is reasonable to suggest that P. robustus and P. boisei represent separate species, but this is not completely conclusive. The degree of anatomical variability observed in P. boisei is greater than that in P. robustus, but this is likely the result of a more plentiful fossil record (Klein, 1999).
It should be noted here that the robustness of Paranthropus refers only to the dentition and related chewing adaptations; body size in this genus was actually very small. In general, the postcranial skeleton of P. boisei was most similar to A. africanus, with similar limb proportions and a similar degree of sexual dimorphism.
P. boisei is very well-represented in the fossil record. It co-existed with early members of the genus Homo in east Africa for several hundred thousand years and is actually more numerous in the fossil record. However, it has been suggested that this is due to taphonomic factors and is not a measure of evolutionary success (Klein, 1999). Ultimately, the cause for the extinction of Paranthropus is unknown. It is possible that climate and ecological changes that also had major impacts on the evolution of the genus Homo were too much for the highly specialized hominids to cope with. However, there is no empirical evidence to support this.
PHYLOGENETIC RELATIONSHIPS OF THE AUSTRALOPITHECINES
Assessing evolutionary relationships based solely on fossil specimens is exceedingly difficult. This is especially true when many of the relevant fossil specimens are highly fragmentary and soft tissues are never preserved. Studies of ancient DNA sequences have been utilized to elucidate evolutionary relationships of the most recent hominid species (e.g. Krings et al., 1997; Ovchinnikov et al., 2000). However, the age of the australopithecines and the poor preservation of the DNA molecule prohibits any similar molecular investigations for earlier hominids. Thus, evolutionary relationships must be inferred solely from morphological differences observed on fossils.
Not surprisingly, there is much disagreement among paleoanthropologists with respect to reconstructing phylogenetic relationships for the australoptihecines. Furthermore, the discovery of new fossil specimens that are unexpected often cause dramatic re-organizations of hominid phylogenies. In addition to this, some new fossils are so out of line with current phylogenies that they cannot be positioned anywhere sensibly on phylogenetic trees and are often left aside with question marks accompanying them (for example: Orrorin tugenensis, Sahelanthropus tchadensis and Kenyanthropus platyops). Finally, paleoanthropologists are people with egos (often large ones) and, not surprisingly, often place their recently discovered fossil specimens at points on the trees which are thought to be the most crucial in the grand scheme of human evolution (being ancestral to the genus Homo for example). For all of these reasons, reconstructing hominid phylogenies is extremely problematic, but still a very necessary task if one wishes to comprehend the evolution of the australopithecines.
One view of australopithecine evolution does not see the robust group as a valid taxonomic unit and retains them in Australopithecus rather than assigning them to a separate genus (see Conry, 1997). A phylogenetic tree representing this view is shown below (Fig. 10).

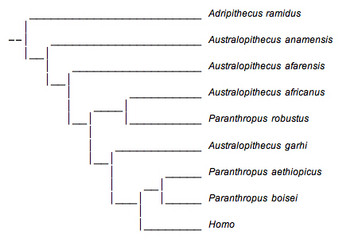
FIGURE 10. This phylogenetic tree presents a view of australopithecine evolution that requires parallel evolution of the robust species in east and south Africa. © 2007 Paul Szpak
One of the most important implications of this reconstruction is that it implies a great degree of parallel evolution for Paranthropus robustus and P. boisei. Although they are referred to as Paranthropus in the tree shown here, most who support this view would refer to these species as: Australopithecus aethiopicus, A. bosei and A. robustus, respectively. This is due to the fact that if P. robustus and P. boisei did not emerge from a common ancestor, the group Paranthropus would be polyphyletic and an invalid evolutionary unit. It is only for the sake of simplicity and consistency that the name Paranthropus is retained in this figure.
In this reconstruction, A. africanus and P. robustus are sister groups, and P. aethiopicus and P. boisei are sister groups. Thus, the hyper-masticatory adaptation of the robust australopithecines would have had to evolve independently in eastern and southern Africa. This is not impossible, especially if similar climatic conditions resulted in similar changes in the availability of food resources in both regions. However, there are several problems with this interpretation.
This view is based largely on geographical locality rather than analyses of morphological characteristics. It ignores the overwhelming number of similarities between P. boisei and P. robustus which were discussed earlier. Furthermore, it is desirable to reconstruct phylogenetic trees which require traits to develop with the fewest number of changes. A very extensive examination of a large number of morphological traits revealed that this phylogenetic tree was not the most parsimonious and suggested that Paranthropus was in fact a meaningful evolutionary unit and did constitute a monophyletic group (Strait et al., 1997).

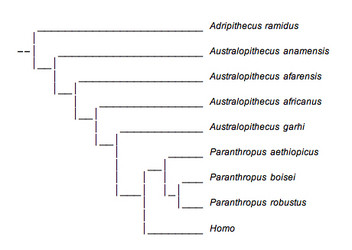
FIGURE 11. This phylogenetic tree presents Paranthropus as a monophyletic group and is in accordance with recent analyses. © 2007 Paul Szpak
A more parsimonious reconstruction of australopithecine evolution presents Paranthropus as a monophyletic group and is shown above (Fig. 11). This tree is similar to one arrived at by Strait et al. (1997), but has incorporated several more recently discovered specimens. This reconstruction is better supported by fossil evidence, but there are still several problems. First, the position of A. anamensis on both phylogenetic trees is very uncertain due to the lack of fossil material available for analysis. It occupies this position simply because of its age and because of a few dental characteristics shared with A. afarensis that were discussed earlier. The same applies to A. garhi.
It is equally difficult to determine the ancestry of the australopithecines. Presently, it seems reasonable to suggest that Adripithecus ramidus is the most likely candidate for being ancestral to the australopithecines based on its age and morphological similarities to Australopithecus anamensis and A. afarensis, but additional fossil evidence may change this view dramatically. For instance, the phylogenetic implications of the recently discovered Sahelanthropus tchadensis have yet to be fully explored.
Determining which species of australopithecine (if any) is ancestral to the genus Homo is a question that is a top priority for many paleoanthropologists, but one that will likely elude any conclusive answers for years to come. Nearly every possible species has been suggested as a likely candidate, but none are overwhelmingly convincing. Presently, it appears that A. garhi has the potential to occupy this coveted place in paleoanthropology, but the lack of fossil evidence is a serious problem. Another problem presents itself in the fact that it has been very difficult to assess which hominid represents the first member of the genus Homo. Without knowing this, it is not possible to determine which species of australopithecine may have been ancestral to Homo.
Whether or not additional fossil evidence will help to resolve these issues is unclear. As more specimens representing current species are discovered, a more complete picture of their anatomy and the variability present within each species will certainly add some resolution to our understanding of human origins. However, it is often the case that new fossil specimens only serve to add to the confusion.
GLOSSARY
Ardipithecus: The most well-known species of hominid pre-dating the australopithecines. Was most likely bipedal and may have been ancestral to the australopithecines.
Australopithecine: Members of the genus Australopithecus and Paranthropus are commonly referred to as australopithecines. Often, Paranthropus is referred to as the ‘robust’ clade and Australopithecus as the ‘gracile’ clade due to their dental characteristics.
Biped (Bipedal Locomotion): An organism that walks on two legs habitually.
Brachial Index: Measure expressing relative length of the forearm and upper arm, (Radius Length / Humerus Length) x 100
C3 & C4 Plants: The majority of plants on earth are C3 plants. C4 plants are generally grasses. They each practice a slightly different type of photosynthesis which can be determined through stable isotope analysis.
Catalogue Number: Fossil specimens are designated by a unique catalogue number by which they are usually refered to in the literature. For example, the Black Skull (Paranthropus aethiopicus) is designated KNM-WT 17000. KNM designates Kenya National Museum (where it is housed), WT designates West Turkana (the site at which this specimen was found) and 17000 is its catalogue number at the museum.
Cranial Capacity: Volume of the inside of the neurocranium, often used as an equivalent for brain volume.
Folivore: A leaf-eating animal.
Foramen magnum: Hole in the base of the cranium where the spinal chord enters.
Frugivore: A fruit-eating animal.
Hominidae: The family which contains humans and their extinct relatives.
Ilium: Part of the pelvis.
Intermembral index: Measure of arm length over leg length (Humers + Radius / Femur + Tibia) x 100. This measure can be used to assess the type of locomotion practice by a fossil specimen.
Lithic: Tools manufactured from stone.
Masseter: Muscle that partly controls the mandible.
Morphology: The study of the physical form and shape of an organism.
Nuchal Crest: Crest on the posterior portion of the cranium for muscle attachment.
Orrorin: Recently discovered specimen pre-dating the australopithecines that may have been bipedal. Its current status is hotly debated.
Paleoanthropology: The study of human ancestors.
Paleoecology: The reconstruction of past ecological conditions.
Pan: The genus which contains modern species of chimpanzees.
Pongid: Refers to the great apes with the exception of the Hominidae.
Pongo: Te genus which contains the modern orang-utan.
Piltdown Man: Possibly the most famous hoax in the history of science. Discovered in England, it consisted of the cranium of a modern human and the mandible of an orangutan. Radiocarbon dating later demonstrated this specimen to be a hoax.
Postcanine Dentition: Refers to the teeth posterior to the canines, namely the premolars and molars.
Postcranial: Parts of the skeleton not associated with the cranium. The limbs and bones of the trunk constitute the postcranial skeleton.
Proximal Condyle: Surface on the femur for articulation with the tibia.
Quadruped: Species that walk on four legs.
Ramapithecus: Initially thought to be ancestral to humans (1960s), was later found to be closely allied with the modern orangutan (Pongo pygmaeus).
Sahelanthropus: A recently discovered genus that may be a very early hominid from Chad (~7.0 Mya) that may have been bipedal.
Sagittal Crest: A bony ridge running anterior-posteriorly down the cranium. This crest facilitates attachment of the jaw muscles and is prominent on some species of australopithecine. It is sometimes referred to as a sagittal keel.
Sexual Dimorphism: Variation in size between males and females.
Stable Isotope Analysis: Elemental analysis used to study the molecular composition of organic materials (bones and teeth for example). Can provide great insight into diet and ecology.
Suspensory: Species that move through the trees by hanging, such as orang-utans.
Taphonomy: Studies the processes that cause decay in organic materials and cause depositional contexts of fossils.
Taung Child: A very famous juvenile specimen of Australopithecus africanus discovered by Raymond Dart in South Africa. It was the first australopithecine to be described (1925).
Taxonomy: The methodology through which organisms are classified.
Temporalis Muscle: Muscle controlling chewing (mastication).
USO (Underground Storage Organ): Underground root, corm or tuber that is rich in starch.
Zooarchaeology: The archaeological analysis of faunal remains.
Zygomatics: What might be referred to commonly as the ‘cheek’ bones.
Information on the Internet
- Becoming Human (Institute of Human Origins) Comprehensive page produced by the IHO with a frequently updated news section.
- Institute of Human Origins (Arizona State University) Information about the institute, its members, field schools, etc.
- The Paleoanthropology Society International society for those interested in paleoanthropology.
- The Evoluton of Man (BBC News) BBC's human origins related take in their prehistoric life series.
- Human Evolution (Minnesota State University E Museum) Virtual museum exhibit that addresses prominent individuals and fossil specimens in human evolution.
- 3D Reconstructions of Fossils (U.C. Santa Barbara) Interactive three dimensional reconstructions of hominid skulls (P.H. Walker and E.H. Hagen).
- Human Evolution News (Science Daily) Frequently updated news related to human evolution.
- The Record of Human Evolution (National Center for Science Education) Brief outline of human evolution by Eric Delson (a prominent paleoanthropologist associated with the NYCEP).
- The Koobi Fora Research Project Outlines past and ongoing research at the important site of Koobi Fora (East Africa).
- The Panda's Thumb General discussion about evolutionary theory.



 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site