The larva of Austramphilina elongata, which has been studied in greatest detail, is about 160 to 219 microns long. It is ciliated and the only large area lacking cilia is the posterior end bearing the hooks. Ten hooks, six of them halberd-shaped with curved and sharply pointed blades, and four with serrate blades, are arranged in a circle at the posterior end. Three types of frontal glands open at and near the anterior end. Two excretory pores are located postero-laterally, each connected to an excretory duct with three flame bulbs (Fig. 1) (Rohde and Georgi, 1983; Rohde, 1986).

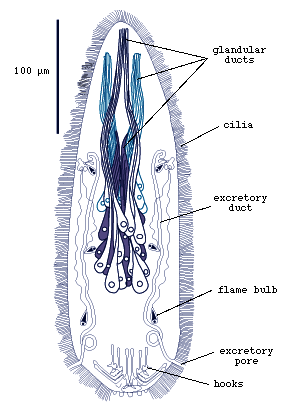
Figure 1. Larva of Austramphilina elongata, showing cilia, hooks, penetration glands and protonephridial system (redrawn from Rohde, 1986).
About 38 transverse muscle bands are distributed between the anterior and posterior ends, and sensory papillae that can be stained with silver-nitrate are clustered mainly at the anterior end (Fig.2).

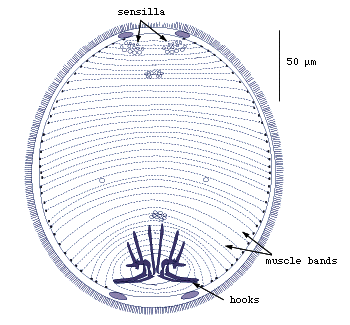
Figure 2. Larva of Austramphilina elongata showing sensilla, hooks and transverse muscle bands (redrawn from Rohde and Georgi, 1983).
Sensory receptors are of several types, one uniciliate with microvilli, one with four short cilia, and several without cilia and either with or without a basal body (Fig.3) (Rohde, Watson and Garlick, 1986).

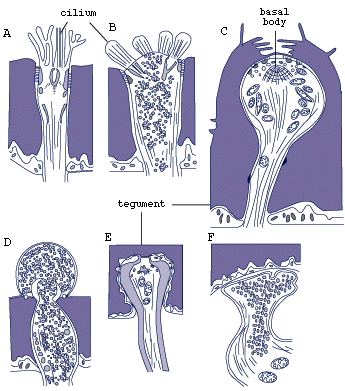

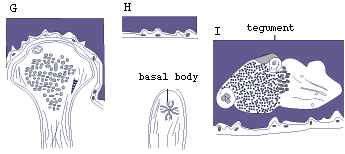
Figure 3. Sensory receptors of larva of Austramphilina elongata based on electron-microscopic studies (redrawn from Rohde, Watson and Garlick, 1986).
The ciliated epidermis lacks lateral cell membranes, i.e., it is a syncytium. Cilia have horizontal cross-striated rootlets but lack vertical ones (Fig.4) (Rohde and Georgi, 1983).

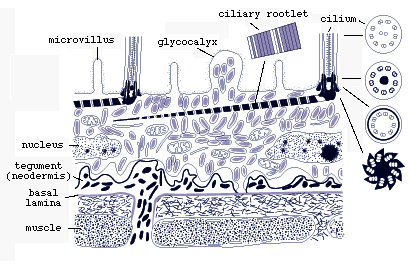
Figure 4. Diagrammatic longitudinal section through surface layers of larval Austramphilina elongata based on electron-microscopic studies. Note the ciliated epidermis overlying the tegument (neodermis) with its basal lamina. The perikarya (nucleated parts of the cells) of the tegument are "insunk", i.e., they are located deep in the tissue. The ciliated epidermis is shed when the larva penetrates into a crayfish, and the tegument becomes the surface layer of the juvenile and adult (redrawn from Rohde and Georgi, 1983).
The epidermis is based on a thin tegument which is also syncytial, its nucleated parts located deep in the interior of the larva (Fig.4). In the ciliary tips, the number of microtubules decreases and they close in around a short dense rod (Fig.5).

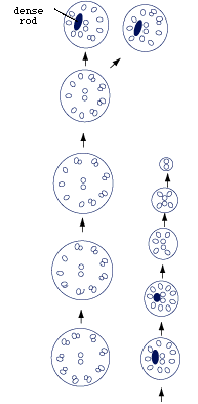
Figure 5. Diagrammatic cross-sections through epidermal cilium of larval Austramphilina elongata. Note dense rod near tip of cilium, and gradual reduction in the number of microtubules in the cilium towards the tip (redrawn from Rohde, 1986).
The secretion of the frontal glands is formed by Golgi complexes alone or by Golgi complexes and microtubules (Fig.6) (Rohde, 1986).

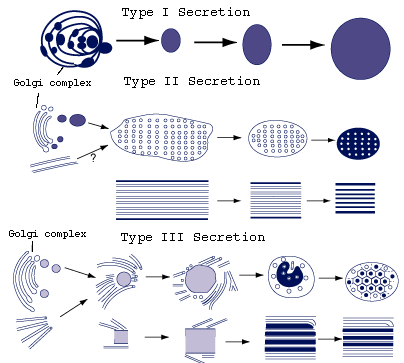
Figure 6. Formation of three types of secretion of larval Austramphilina elongata. Note: type I secretion formed by Golgi complex, types II and III by Golgi complex and microtubules (redrawn from Rohde, 1987).
The proximal parts of the protonephridial system consists of bundles of three flame bulbs, each formed by a terminal cell and a branch of a proximal canal cell that supplies all three terminal cells of a bundle (Fig.7).

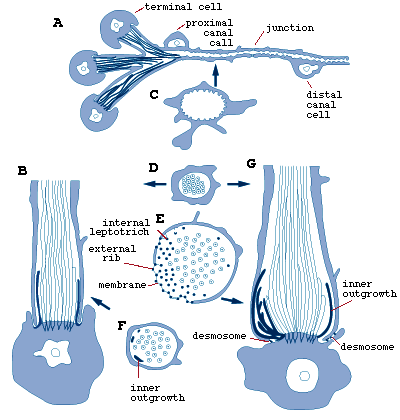
Figure 7. Larval Austramphilina elongata, structure of protonephridium (A and C, C is a cross-section through the proximal canal), and development of flame bulb (B is a slightly earlier stage than G. D, E and F are cross-sections through the parts indicated by arrows) (redrawn from Rohde and Watson, 1988).
Each terminal cell carries a bundle of cilia, the flame, and its rib-like cytoplasmic processes interdigitate with those of the proximal canal cell, with which they are connected by a filtration membrane of extracellular matrix, forming the filtration apparatus or weir. The inner ribs are continuous with the terminal cell, and the outer ribs are continuous with the proximal canal cell. The terminal cell also possesses many internal leptotriches (cytoplasmic outgrowths into the lumen of the flame bulb) and some external leptotriches (cytoplasmic outgrowths into the surrounding tissue spaces).The proximal canal cell is connected to the distal canal cell by a septate junction (Fig.7). The wall of both the proximal and distal canal possess short microvilli. During development of the flame bulbs, internal cytoplasmic processes of the terminal cells grow into the space between the outermost cilia of the flame and the continuous cylinder growing from the proximal canal cell around the flame, and at the points of contact between the cytoplasmic processes and the cylinder external ribs are formed (Fig.7).


 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site