Sirenidae
Sirens
Allan Larson

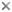
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxRelationships among the three extant species of the family Sirenidae assuming monophyly of the genus Siren.
Introduction
Salamanders of the family Sirenidae do not metamorphose and retain the appearance of an aquatic larva throughout life. They are long and slender, with external gills, gill slits, small forelimbs and no hindlimbs. The complete absence of hindlimbs distinguishes sirens from all other salamanders. Sirens are often described as eel-like in appearance.
Sirens inhabit shallow water in swamps, ditches and ponds, where muddy substrates and dense growth of weeds produce favored habitats.
Sirens are often nocturnal in their activities and spend the day under cover objects, burrowing in mud and weeds. Glands in the skin can create a moisture-retaining seal over the body, permitting sirens to aestivate in mud burrows when the shallow water of their habitats evaporates. When active, sirens feed on a variety of invertebrate prey and plants, and the greater siren also eats small fish.
Breeding occurs in winter or spring, and larvae hatch in spring. Mating has not been observed for sirens, but fertilization of the eggs is assumed to be external because the cloacal glands used by internally fertilizing salamanders are absent in sirens (see "Characteristics" section below). Eggs are guarded by females until they hatch.
Geographic distributions of the dwarf siren (Pseudobranchus striatus) and greater siren (Siren lacertina) center around the Florida peninsula and include adjacent areas in southern and coastal Georgia and South Carolina. The greater siren also extends north along the Atlantic coast through Virginia and west along the Gulf coast to Alabama. The intermediate siren (Siren intermedia) inhabits Atlantic and Gulf coastal regions from North Carolina to Texas and northern Mexico, and also extends north through Arkansas, Missouri, Tennessee, Kentucky, Illinois, Indiana and Michigan. Fossils indicate that the Sirenidae formerly had a broader geographic distribution in North America than currently observed, extending at least to Wyoming. No fossil sirenids are known from outside North America.
Characteristics
Diagnosis
Sirens are permanently aquatic and larval in form with a finlike tail, large external gills, 1 or 3 pairs of gill slits, small eyes and no eyelids. Forelimbs are relatively small (although larger than those of amphiumas) and have four digits. Absence of hindlimbs distinguishes sirens from all other salamanders. Sirens can aestivate in mud burrows by secreting mucus around the body to protect it from desiccation. Sirens have highly elongated bodies and are sometimes mistaken for eels.
Detailed Characteristics of the Sirenidae
The morphological characters given below are the ones standardly used to diagnose the salamander family Sirenidae and to assess its phylogenetic relationships to other salamanders. The individual characteristics are in many cases shared with other salamanders and should not be interpreted as synapomorphies of the Sirenidae. Absence of characteristics found in other salamanders is noted where it is important for distinguishing sirens from other salamanders and/or determining their relationships to other salamanders. These characteristics were assembled from a large number of original sources by Duellman and Trueb (1986), Larson (1991) and Larson and Dimmick (1993).
Metamorphosis is absent in the Sirenidae, leading to a number of paedomorphic characteristics in adults.
Morphology of the skull
Premaxilla consists of separated, paired bones. Bilaterally paired nasal bones each ossify from a single, medially-positioned anlage; long posterior processes of the premaxillae extend lateral to the nasal bones, which abut each other. Maxillary bones are present but small. Septomaxillary bones are absent. Lacrimal bone is absent. Quadratojugal bone is absent. Pterygoid bones are present but small. Internal carotid foramina are absent from parasphenoid bones. The angular bone is fused with the mandible. Ear bones include a detached columella but no operculum. Replacement of vomerine teeth proceeds laterally in parallel to the maxillary teeth. Teeth do not have a distinct crown and pedicel. Origin of the levator mandibulae anterior superficialis muscle occurs on the side of the skull and does not include the exoccipital. Eyelids are absent.
Inner ear
A basilaris complex is present in the inner ear. The recessus amphibiorum is oriented horizontally in the inner ear. The otic sac is bulbar and partially vascularized. The amphibian periotic canal lacks fibrous connective tissue. The periotic cistern is large. The periotic cistern protrudes into the fenestra.
Hyobranchial structures
The first hypobranchial and first ceratobranchial (alternatively homologized as the first ceratobranchial and first epibranchial, respectively) exist as separate structures. The second ceratobranchial (alternatively homologized as the second epibranchial) comprises four elements. Lungs are present but the ypsiloid cartilage is absent. Larvae have one pair (Pseudobranchus) or three pairs (Siren) of gill slits.
Characteristics of the trunk and vertebral column
The scapula and coracoid occur as separate bones of the pectoral girdle. Vertebral centra are amphicoelous. Ribs are bicapitate. Spinal-nerve foramina are present in neural arches of vertebrae for all spinal nerves except those exiting between the atlas and first trunk vertebra. The pubotibialis and puboischiotibialis are present as separate muscles. Anterior glomeruli of the kidney are well developed.
Reproductive characters
Fertilization has not been observed but is presumed to be external. Ciliated epithelium is absent from the cloacal tube and anterior cloacal chamber of females. Epidermal lining is present in the anterior cloacal chamber of females. Evaginations are absent from the dorsolateral walls of the male cloacal tube. Anterior ventral glands are absent from the cloacae of females. No spermathecae are present in the female cloacal chamber. Glands secreting into the dorsal walls of the female cloaca are absent. Anterior ventral glands are absent from male cloacae. Posterior ventral glands are absent from male cloacae. Kingsbury's glands are absent from male cloacae. Dorsal pelvic glands are absent in males. Lateral pelvic glands are absent in males. Glands secreting into the male cloacal orifice are absent. Parental care of eggs is by females.
The diploid number of chromosomes is high (46 for Siren intermedia, 52 for Siren lacertina and 64 for Pseudobranchus striatus; see Morescalchi, 1975).
Classification
The salamander family Sirenidae is the sole member of the caudate suborder Sirenoidea. Sirens are sufficiently different from other salamanders that they sometimes have been considered a separate taxonomic order (named Meantes or Trachystomata) of amphibians. Phylogenetic studies have disagreed on the exact placement of sirens, but the best current hypothesis (see below) is that they are the sister taxon to all remaining salamanders. They are clearly closer to the salamanders than to any other extant amphibians, and their placement in the order Caudata therefore is appropriate.
Discussion of Phylogenetic Relationships
Unique morphological characteristics clearly establish monophyly of the Sirenidae (Duellman and Trueb, 1986) and monophyly is supported also by molecular data (Larson, 1991). The phylogeny presented here assumes monophyly of the genus Siren, but additional molecular studies are needed to test this hypothesis.
The best current hypothesis of salamander family relationships based upon molecular and morphological data (Larson and Dimmick, 1993) places the Sirenidae as the sister taxon to all remaining salamanders. This relationship was suggested earlier by Duellman and Trueb (1986) based upon morphology. The Sirenidae therefore appears to represent an ancient lineage that has no close phylogenetic relatives among extant organisms.
References
Duellman, W. E. and L. Trueb. 1986. Biology of Amphibians. McGraw-Hill, New York.
Estes, R. 1981. Gymnophiona, Caudata. Handbuch der Paläoherpetologie 2:1-115.
Larson, A. 1991. A molecular perspective on the evolutionary relationships of the salamander families. Evolutionary Biology 25:211-277.
Larson, A. and W. W. Dimmick. 1993. Phylogenetic relationships of the salamander families: A analysis of congruence among morphological and molecular characters. Herpetological Monographs 7:77-93.
Morescalchi, A. 1975. Chromosome evolution in the caudate Amphibia. Evolutionary Biology 8:339-387.
Title Illustrations

| Scientific Name | Siren intermedia |
|---|---|
| Location | Berkeley Co., South Carolina |
| Copyright | © 1996 Greg Sievert |
About This Page
David Heyse, Todd Jackman and Greg Sievert contributed to the preparation of this Tree of Life page.
Allan Larson

Washington University, St. Louis, Missouri, USA
Page copyright © 1996 Allan Larson
All Rights Reserved.
Citing this page:
Larson, Allan. 1996. Sirenidae. Sirens. Version 01 January 1996 (under construction). http://tolweb.org/Sirenidae/15454/1996.01.01 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site