Pucciniomycetes
Merje Toome and M. Catherine Aime

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Pucciniomycetes is a diverse class of basidiomycete fungi that contains the vast majority (ca. 8000) of the species in subphylum Pucciniomycotina. Almost all of the organisms in Pucciniomycetes are parasites of plants, insects, or other fungi. The most species-rich group of the Pucciniomycetes are important and well-known plant pathogens called rusts (Pucciniales), named for the typically rusty color of their spores (Fig 1). Rust fungi are obligate parasites of vascular plants with highly complex life cycles requiring the production of up to five different spore stages on two different host plants (Cummins and Hiratsuka, 2003). Species of Pucciniales cause some of the most devastating plant diseases and therefore have been studied in greater detail than other Pucciniomycotina. Approximately 7800 species of Pucciniales have been described, i.e. roughly a quarter of all known species in Basidiomycota and ca. 8% of all described Fungi (Kirk et al., 2008).


Figure 1. Symptoms on barley leaf caused by leaf rust (Puccinia hordei). The orange pustules contain thousands of asexually produced urediniospores. © 2009 Merje Toome
The remaining ca. 200 species in the Pucciniomycetes belong to four orders and are of little economic importance. The largest among these is Septobasidiales, of which more than 150 species are known, and which contains the only entomopathogenic (e.g., pathogens of insects) species in Pucciniomycotina. Septobasidiales parasitize scale insects that feed on trees, forming dense fungal mats that cover the insects on their hosts (Couch, 1938).
The next two largest orders contain either species parasitic on mosses and ferns (Platygloeales, ca. 20 species) or parasites that alternate between plant roots and rust fungi (Helicobasidiales, ca. 17 species). Some species in Helicobasidiales have been the focus of ecological studies to determine their potential as biocontrol agents against rusts, but very little is known about the other species in these orders. The fifth order, Pachnocybales, contains a single species, Pachnocybe ferruginea, which appears to be a saprobe and therefore differs significantly from all other Pucciniomycetes by having a non-parasitic life style (Aime et al., 2006).
Characteristics
The dikaryon (i.e., containing two haploid nuclei per cell) is the dominant phase in Pucciniomycetes and only one of the orders, Septobasidiales, is known to have a monokaryotic (e.g., containing a single haploid nucleus per cell) yeast stage, a feature that is otherwise prominent in Pucciniomycotina. Unlike many of the other basidiomycetes, including some Pucciniomycotina species, the members of Pucciniomycetes lack clamp connections on their hyphae (Bauer et al., 2006). Production of asexual spores (Fig. 2) is often well developed, especially among rusts where one species has been observed to produce five morphologically distinct types of asexually produced spores (Bruckart et al., 2010).


Figure 2. Different spores produced by rust on rose (Phragmidium fusiforme). Yellow rounded thick-walled spores are urediniospores and dark multicelled spores are teliospores. Both spore types are dikaryotic. © 2011 Merje Toome
In general, sexual reproduction takes place via the formation of basidiospores. These are formed on four-celled metabasidia (also known as phragmobasidia because they are septate), which develop from a probasidium. A probasidium is generally a specialized region of the basidium where karyogamy occurs and may often be a thick-walled resting spore (i.e., a teliospore). Meiosis occurs in the metabasidium and is followed immediately by the formation of four haploid basidiospores of two different mating types. The basidiospores germinate and form a monokaryon. When two monokaryons of different mating types fuse, a new dikaryon forms via plasmogamy which may give rise to asexual reproductive stage(s) before eventually producing probasidia which start the cycle over again (Cummins and Hiratsuka, 2003). Pachnocybe ferruginea is again the exception for Pucciniomycetes since rather than phragmobasidia, it produces holobasidia (i.e., basidia that are not septate) (Kropp and Corden, 1986). Pucciniales are known to be heteroecious, i.e., they are obligate parasites that alternate between two unrelated hosts during different stages of their life cycle. Members of Helicobasidiales also need to alternate between two hosts; the dikaryon parasitizes plant stems and roots, whereas the monokaryotic stage parasitizes relatives in the Pucciniales (Lutz et al., 2004). Interestingly, the character of heteroecism appears to have arisen only once in Fungi outside of Pucciniomycetes, in the unrelated chytrid genus Coelomomyces (Blastocladiales, Blastocladiomycetes).
The most important ultrastructural character of Pucciniomycetes is that of a simple septal wall with a central pore that in many species has a pulley-wheel-shaped plug (Swann et al., 2001). In addition to a simple septum, Pucciniomycetes share the character of all species in Pucciniomycotina in having layered discoid spindle pole bodies. These are microtubule organizing centers, embedded in the nuclear envelopes. The spindle pole body morphology is highly variable between different groups. In Pucciniomycetes it is inserted in a pore of the nuclear envelope (Bauer et al., 2006). In Septobasidiales and Pachnocybales, the presence of unique membrane complexes, termed microscala, has also been reported (Swann et al., 2001).
Discussion of Phylogenetic Relationships
Before the availability of DNA sequence data, Pucciniomycetes were placed in various positions on the fungal tree of life. For instance, based on some of their ultrastructural characters (e.g., lack of clamp connections) and parasitic life style, Pucciniales and their relatives were often thought to represent an early diverging lineage of Basidiomycota. Phylogenetic studies based on ribosomal DNA (rDNA) have shown that rusts and their closest relatives in Pucciniomycetes are a derived group within the Pucciniomycotina (Aime et al., 2006). However, the relationships between the orders in Pucciniomycetes and even between Pucciniomycetes and other classes in Pucciniomycotina remain unresolved and need additional phylogenetic studies.
References
Aime, M. C., P. B. Matheny, D. A. Henk, E. M. Frieders, R. H. Nilsson, M. Piepenbring, D. J. McLaughlin, L. J. Szabo, D. Begerow, J. P. Sampaio, R. Bauer, M. Weiß, F. Oberwinkler, and D. S. Hibbett. 2006. An overview of the higher-level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit rDNA sequences. Mycologia 98: 896–905.
Bauer, R., Begerow, D., Sampaio, J.P., Weiss, M., Oberwinkler, F. 2006. The simple-septate basidiomycetes: a synopsis. Mycological Progress 5 (1): 41–66.
Bruckart, W. L., Eskandari, F. M., Berner, D. K., Aime, M.C. 2010. Life cycle of Puccinia acroptili on Rhaponticum (= Acroptilon) repens. Mycologia 102 (1): 62-68.
Couch, J.N. 1938. The genus Septobasidium. The University of North Carolina Press, Chapel Hill NC. 480 pp.
Cummins, G.B., and Y. Hiratsuka. 2003. Illustrated Genera of Rust Fungi. APS Press, St. Paul, MN, 225 pp.
Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lücking, T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. McLaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y.-C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. Hyde, J. E. Ironside, U. Kõljalg, C. P. Kurtzman, K.-H. Larsson, R. Lichtwardt, J. Longcore, J. Miądlikowska, A. Miller, J.-M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schüßler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiß, M. M. White, K. Winka, Y.-J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–547.
Kirk, P. M., Cannon, P. F., Minter, D. W., Stalpers, J. A. 2008. Dictionary of the Fungi (10th ed.). Wallingford, UK: CABI. p. 581.
Kropp, B. R., Corden, M. E. 1986. Morphology and taxonomy of Pachnocybe ferruginea. Mycologia 78 (3): 334-342.
Lutz , M., Bauer, R., Begerow, D., Oberwinkler, F. 2004. Tuberculina — Thanatophytum/Rhizoctonia crocorum — Helicobasidium: a unique mycoparasitic-phytoparasitic life strategy. Mycological Research 108: 227-238.
Swann, E. C., Frieders, E. M., McLaughlin, D. J. 2001. Urediniomycetes. In: D. J. McLaughlin, E. J. McLaughlin, and P. Lemke, eds., The Mycota. Vol. VII. Part B, Systematics and Evolution. Springer-Verlag, Berlin, p. 37-56.
Title Illustrations

| Scientific Name | Gymnosporangium juniperi-virginianae and Juniperus virginiana |
|---|---|
| Location | United States |
| Specimen Condition | Live Specimen |
| Life Cycle Stage | Fruiting Bodies |
| Body Part | Telial-stage horns of the cedar-apple rust pathogen |
| Source | #5028064 |
| Source Collection | Bugwood Network/Forestry Images |
| Image Use |
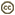 This media file is licensed under the Creative Commons Attribution License - Version 3.0. This media file is licensed under the Creative Commons Attribution License - Version 3.0.
|
| Copyright |
© Joseph O'Brien

|
| Scientific Name | Helicobasidium longisporum on roots and basal parts of stems of Aster sp. cult. |
|---|---|
| Location | Germany, Baden-Württemberg, Stuttgart |
| Comments | on roots and basal parts of stems of Aster sp. cult. |
| Specimen Condition | Live Specimen |
| Identified By | M. Lutz |
| Life Cycle Stage | Sterile stage (Thanatophytum) of the phytoparasitic teleomorph |
| Collection | 2000 |
| Image Use |
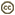 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2000
Matthias Lutz

|
| Scientific Name | Eocronartium muscicola |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | D.J. McLaughlin |
| Life Cycle Stage | fruitbodies on moss host |
| Collection | MIN 864444 |
| Collector | David J. McLaughlin |
| Copyright | © 2001 Bell Museum of Natural History |
About This Page
Development of this page was facilitated by the "Deep Hypha" Research Coordination Network and the "Assembling the Fungal Tree of Life" project (NSF award DEB-0732968).
Merje Toome

Purdue University
M. Catherine Aime

Louisiana State University Agricultural Center
Correspondence regarding this page should be directed to Merje Toome at
MToome@purdue.edu
and M. Catherine Aime at
maime@agcenter.lsu.edu
Page copyright © 2012 Merje Toome and M. Catherine Aime
 Page: Tree of Life
Pucciniomycetes.
Authored by
Merje Toome and M. Catherine Aime.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Pucciniomycetes.
Authored by
Merje Toome and M. Catherine Aime.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 21 February 2008
- Content changed 30 January 2012
Citing this page:
Toome, Merje and M. Catherine Aime. 2012. Pucciniomycetes. Version 30 January 2012 (under construction). http://tolweb.org/Pucciniomycetes/51246/2012.01.30 in The Tree of Life Web Project, http://tolweb.org/










 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site