Oneirodidae
Dreamers
Theodore W. Pietsch

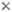
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Oneirodidae is by far the largest, most complex, and certainly the least understood family of the suborder. With 16 genera and 62 species, it contains nearly 40% of all recognized ceratioids. Of the 16 genera, five are currently represented by only one, two, or three adolescent or adult females; only eight are represented by more than a dozen females. Males have been described for only seven genera, while larvae are known for only eight. But despite the rareness of most recognized taxa, new oneirodids are continually being described.
Characteristics
Diagnosis
Metamorphosed females of the family Oneirodidae are distinguished from those of all other ceratioid families by having the following combination of character states: supraethmoid present; frontals widely separated; parietals present; sphenotic overlapped by anterolateral projection of pterotic; metapterygoid and mesopterygoid present; hypohyals 2; branchiostegal rays 6 (2 + 4); angular and preopercular spines absent; jaws equal anteriorly; postmaxillary process of premaxillae absent; anterior-maxillomandibular ligament well developed; pharyngobranchial IV absent; a single ossified basibranchial; ceratobranchial teeth absent; epibranchial I free, not bound to wall of pharynx by connective tissue; proximal one-fourth to one-half of ceratobranchial I bound to wall of pharynx, distal three-quarters to one-half free; distal end of ceratobranchial I free, not bound by connective tissue to adjacent ceratobranchial II; proximal one-quarter to one-half of ceratobranchials II-IV not bound together by connective tissue; epurals absent; hypural plate entire, without posterior notch; pterygiophore of illicium bearing an ossified remnant of second cephalic spine; escal bulb and central lumen present, esca without tooth-like denticles; pectoral radials 3; dorsal-fin rays 4-8; anal-fin rays 4-7, very rarely 3; pectoral-fin rays 14-30; caudal-fin rays 9 (2 simple + 4 bifurcated + 3 simple); ovaries paired; one or two small pyloric caecae present or absent.


Lateral view of esca of Oneirodes macrosteus. © 2005 E. A. Widder
Although not characteristic of all members of the family, the following additional features are important in differentiating the Oneirodidae: each frontal with a prominent ventromedial extension (absent in Lophodolos); sphenotic spines usually present (absent in Chaenophryne); pterosphenoid usually present (absent in Lophodolos); hyomandibular usually with a double head (single head in Bertella); opercle bifurcate, dorsal fork supported by a single rib (except in larval females of Danaphryne); anterior subopercular spine usually absent (blunt projection present in most specimens of Chaenophryne, adolescent females of Lophodolos, and some larvae and males of Dolopichthys and Pentherichthys); quadrate and articular spines usually well developed (small to rudimentary in Chaenophryne); lower jaw usually with a well-developed symphysial spine (small, blunt to absent in Chaenophryne, absent in Pentherichthys); pharyngobranchial I usually absent (present in Spiniphryne and Oneirodes); pharyngobranchial II usually well developed and heavily toothed (reduced and toothless in Bertella, Microlophichthys, and some species of Oneirodes; absent in Pentherichthys and Lophodolos); epibranchial teeth usually absent (present on epibranchial I of some species of Oneirodes); ossified posteroventral process of coracoid usually absent (present in Spiniphryne and Oneirodes); pelvic bone usually rod-like, with or without slightly expanded distal tip (triradiate or broadly expanded distally in Chaenophryne; Pietsch, 1975:79, fig. 2); dermal spinules usually absent (well developed in Spiniphryne, minute and widely scattered in Oneirodes).

Free-living males of oneirodids. A Chaenophryne draco-group, 14 mm, ZMUC P92686; B Dolopichthys sp., 12.5 mm, ZMUC P92799; C Microlophichthys andracanthus, 16.5 mm, ZMUC P9293; D Oneirodes sp., 12.5 mm, ZMUC P921016; E Pentherichthys sp., 13 mm, ZMUC P921113. (All after Bertelsen, 1951, © 1951 Bertelsen).
Metamorphosed males are distinguished from those of all other ceratioid families in having the following combination of character states: eyes directed laterally, elliptical in shape, axis short, diameter of pupil greater than that of lens; olfactory organs large, anterior nostrils situated close together and opening forwards; posterior nostrils lateral, usually larger than eye; nasal area usually pigmented, sometimes slightly inflated; jaw teeth absent; dermal spinules of snout absent; posterior end of upper denticular remote from anterior end of pterygiophore of illicium; fin-ray counts as given for metamorphosed females; skin naked (but males of the spiny-skinned oneirodid genus Spiniphryne are unknown); free-living, non-parasitic, with two exceptions, both apparently facultative couplings: a single known attached pair of Leptacanthichthys gracilispinis and another of Bertella idiomorpha (see Pietsch, 2005).
There is no satisfactory combination of features that serve to adequately diagnose oneirodid larvae. As stated by Bertelsen (1951:71), it is “easier in practice to make the identification [of larvae] from the features characteristic of each of the many genera within the family.” To a somewhat lesser extent, the same could also be said for the metamorphosed males and females.
Description
Metamorphosed females highly variable, ranging from short and deep-bodied, more-or-less globular (e.g., Oneirodes and Chaenophryne), to elongate and fusiform (e.g., Dolopichthys and Leptacanthichthys); head usually short, approximately 35% SL or less (30% SL in Dermatias, nearly 50% SL in some species of Chaenophryne); mouth large, opening horizontal to nearly vertical, cleft extending well past eye in most genera (terminating anterior to eye in Spiniphryne, Danaphryne, Phyllorhinichthys, Puck, Ctenochirichthys, and Bertella); jaw teeth slender, recurved, and depressible, those in upper jaw usually somewhat shorter and fewer than those in lower jaw (upper-jaw teeth more numerous than lower in Danaphryne, Microlophichthys, Tyrannophryne, Phyllorhinichthys, Puck, Leptacanthichthys, and Ctenochirichthys); number of premaxillary and dentary teeth highly variable, lower jaw with fewer than 20 (e.g., Phyllorhinichthys and some species of Oneirodes) to nearly 600 (e.g., some species of Dolopichthys); vomerine teeth usually present (lost with growth in Bertella and some species of Dolopichthys, absent in Lophodolos and Pentherichthys); epibranchial I free from wall of pharynx; epibranchials I-IV closely bound together; proximal one-fourth to one-half of ceratobranchial I bound to wall of pharynx, distal three-fourths to one-half free; epibranchial IV and ceratobranchial IV bound to wall of pharynx, no opening behind fourth arch; gill filaments present on proximal tips of epibranchials II-IV, on proximal tip of ceratobranchial I, and full length of ceratobranchials II-IV; pseudobranch absent; length of illicium highly variable, extremely short and nearly fully enveloped by tissue of esca (e.g., Tyrannophryne) to 75% SL (e.g., some species of Oneirodes and Dolopichthys); anteriormost tip of pterygiophore of illicium usually exposed (hidden in Tyrannophryne), emerging at tip of snout from between eyes or more posteriorly; posterior end of pterygiophore usually concealed under skin (protruding on dorsal surface of trunk behind head in Oneirodes); escal bulb simple to highly complex; neuromasts of acoustico-lateralis system located at tips of low rounded cutaneous papillae, pattern of placement as described for other ceratioids (Pietsch, 1969, 1972, 1974a, 1974b).
Color of females in preservative dark brown to black over entire external surface of body (except for escal appendages and distal portion of escal bulb); oral cavity and viscera, except for outer surface of stomach wall, unpigmented. Metamorphosed males with outer pigmentation as for females, except nasal area often unpigmented; subdermal pigmentation of males and larvae highly variable (see Bertelsen, 1951:73).
The largest known female is a 370-mm specimen of Oneirodes heteronema, collected by the Walther Herwig during the 1982 expedition to the mid-Atlantic Ridge. The largest known male measures 16.5 mm.
Distribution
The Oneirodidae is widely distributed throughout the more productive waters of all three major oceans of the world. Unlike some ceratioid families (e.g., Melanocetidae), which appear to be limited by the Arctic and Antarctic Polar Fronts, oneirodids extend northward to at least 66º in the North Atlantic and 62º in the Bering Sea, and southward into the Southern Ocean to at least 65ºS. It is represented in the Caribbean Sea and in the gulfs of Aden, California, and Mexico; but, like all other ceratioids, it has not been collected in the Mediterranean Sea.
Key to Males and Females of Genera of the Oneirodidae
Females
Because several genera are known from very few specimens (only two adolescent females in Tyrannophryne, Chirophryne, and Puck), and for many, larvae and males are unknown, the following keys to females and males, both modified from Bertelsen (1951:74-76) and Pietsch (1974a:30), are tentative and may not include the best diagnostic characters.
1A. Sphenotic spines present; opercle deeply notched posteriorly; pelvic bones rod-shaped, with or without slight distal expansion (go to 2)
1B. Sphenotic spines absent; opercle not deeply notched posteriorly; pelvic bones triradiate or greatly expanded distally (Chaenophryne Regan, 1925)
2A. Pectoral-fin lobe short and broad, shorter than longest pectoral-fin rays (go to 3)
2B. Pectoral-fin lobe long and narrow, longer than longest pectoral-fin rays (go to 10)
3A. Skin covered with close-set dermal spinules (Spiniphryne Bertelsen, 1951)
3B. Skin naked or with minute, widely spaced dermal spinules (visible only with the aid of a microscope in cleared and stained specimens) (go to 4)
4A. Lower jaw with a symphysial spine; caudal rays without internal pigment (go to 5)
4B. Lower jaw without a symphysial spine, ventral margin of dentaries at symphysis concave; caudal rays internally pigmented (Pentherichthys Regan and Trewavas, 1932)
5A. Illicial apparatus emerging near tip of snout, from between frontal bones (go to 6)
5B. Illicial apparatus emerging from dorsal surface of head, between or behind sphenotic spines (Lophodolos Lloyd, 1909)
6A. Depth of caudal peduncle greater than 20% SL (Dermatias Smith and Radcliffe, 1912)
6B. Depth of caudal peduncle less than 20% SL (go to 7)
7A. Dorsal margin of frontal bones strongly convex; subopercle short and broad, ventral end nearly circular (go to 8)
7B. Dorsal margin of frontal bones nearly straight; subopercle long and narrow, ventral end strongly oval (go to 15)
8A. Caudal fin covered with darkly pigmented skin for some distance beyond fin base; anal-fin rays 5, rarely 4 (go to 9)
8B. Caudal fin not covered by darkly pigmented skin except at base; anal-fin rays 4, rarely 5 (Oneirodes Lütken, 1871)
9A. Lower jaw extremely long, extending posteriorly well beyond base of pectoral fin (Tyrannophryne Regan and Trewavas, 1932)
9B. Lower jaw short, terminating anterior to base of pectoral fin (go to 10)
10A. Fewer than 20 teeth in lower jaw; esca with three stout, non-tapering, internally pigmented appendages arising from dorsal surface; smaller specimens (approximately less than 100-mm SL) with a pair of unpigmented leaf-like appendages on snout (Phyllorhinichthys Pietsch, 1969)
10B. More than 25 teeth in lower jaw; esca without stout appendages arising from dorsal surface; no leaf-like appendages on snout (go to 12)
11A. Cleft of mouth extending past eye; length of escal bulb more than one-half length of illicial bone; dorsal end of subopercle broad and rounded (Microlophichthys Regan and Trewavas, 1932)
11B. Cleft of mouth not extending past eye; escal bulb considerably shorter than one-half length of illicial bone; dorsal end of subopercle slender, tapering to a point (Danaphryne Bertelsen, 1951)
12A. Sphenotic, quadrate, and articular spines long, piercing skin; length of pectoral-fin lobe less than 15% SL; pectoral-fin rays 18-21 (go to 13)
12B. Sphenotic, quadrate, and articular spines short, in some specimens not piercing skin; length of pectoral-fin lobe greater than 15% SL; pectoral-fin rays 28-30 (Ctenochirichthys Regan and Trewavas, 1932)
13A. Length of quadrate spine greater than length of articular spine; dorsal profile of frontal bones convex; esca with more than a single appendage, either five separate appendages arising from dorsal surface or three dorsal appendages and a lateral filament; anal-fin rays 4 (go to 14)
13B. Length of quadrate spine less than length of articular spine; dorsal profile of frontal bones nearly linear; esca with a single appendage arising from dorsal surface; anal-fin rays 5-6 (Leptacanthichthys Regan and Trewavas, 1932)
14A. Length of anterior maxillomandibular ligament greater than one-half length of premaxilla, gape of mouth extending beyond eye; subopercle short and broad, upper end rounded; esca without a lateral filament (Chirophryne Regan and Trewavas, 1932)
14B. Length of anterior maxillomandibular ligament less than one-half length of premaxilla, gape of mouth not extending beyond eye; subopercle long and narrow, upper end tapering to a point; esca with a lateral filament (Puck Pietsch, 1978)
15A. Hyomandibula with a single head (Bertella Pietsch, 1973)
15B. Hyomandibula with a double head (Dolopichthys Garman, 1899)
Males
In the following key, slightly modified from Bertelsen (1951:75), it is uncertain how much diagnostic value can be ascribed to the number of denticular teeth and olfactory lamellae; these presumably increase during adolescent development and therefore may be subject to greater individual variation than indicated here.
1A. Pectoral-fin rays 28-30, articulating along dorsal margin of an elongate, pectoral-fin lobe (Ctenochirichthys Regan and Trewavas, 1932)
1B. Pectoral-fin rays 14-27, articulating along posterior margin of a short and broad pectoral-fin lobe (go to 2)
2A. Subopercle short and broad, dorsal end rounded (go to 3)
2B. Subopercle long and slender, dorsal end tapering more or less to a point (go to 4)
3A. Skin between nostrils unpigmented; medial surface of subopercle unpigmented; caudal peduncle without subdermal pigment; anal-fin rays 4, rarely 3 or 5; pectoral-fin rays 13-19 (Oneirodes Lütken, 1871)
3B. Skin between nostrils slightly pigmented; medial surface of subopercle darkly pigmented; caudal peduncle with subdermal pigment; anal-fin rays 5, rarely 4 or 6; pectoral-fin rays 18-23 (Microlophichthys Regan and Trewavas, 1932)
4A. Posterior nostril contiguous with eye; nasal area unpigmented; opercle deeply notched posteriorly; lower denticular with 4-10 teeth (go to 5)
4B. Posterior nostril not contiguous with eye; nasal area pigmented; opercle only slightly concave posteriorly; lower denticular with 17-27 teeth (Chaenophryne Regan, 1925)
5A. Lower denticular with 8-10 teeth; olfactory lamellae 10-11; caudal peduncle with distinct subdermal pigment; caudal-fin rays without internal pigment; pectoral-fin rays 17-22 (Dolopichthys Garman, 1899)
5B. Lower denticular with 4 teeth; olfactory lamellae 18; caudal peduncle with faint subdermal pigment; caudal-fin rays internally pigmented; pectoral-fin rays 21-27 (Pentherichthys Regan and Trewavas, 1932)
References
Bertelsen, E. 1951. The ceratioid fishes. Ontogeny, taxonomy, distribution and biology. Dana Rept., 39, 276 pp.
Garman, S. 1899. Reports on an exploration off the west coasts of Mexico, Central and South America, and off the Galapagos Islands, in charge of Alexander Agassiz, by the U. S. Fish Commission steamer "Albatross," during 1891, Lieut. Commander Z. L. Tanner, U. S.N., commanding. XXVI. The fishes. Mem. Mus. Comp. Zool., 24, 431 pp.
Lloyd, R. E. 1909. A description of the deep-sea fish caught by the R. I. M. S. Ship "Investigator" since the year 1900, with supposed evidence of mutation in Malthopsis. Mem. Indian Mus., Calcutta, 2(3): 139-180.
Lütken, C. F. 1871. Oneirodes eschrichtii Ltk. en ny grønlandsk Tudsefisk. Oversigt over det Kongl. Danske Vidensk. Selsk. Forhandl., 1871: 56-74. [In Danish, French summary in the same volume, pp. 9-18.]
Pietsch, T. W. 1969. A remarkable new genus and species of deep-sea anglerfish (family Oneirodidae) from off Guadalupe Island, Mexico. Copeia, 1969(2): 365-369.
Pietsch, T. W. 1972. A second specimen of the deep-sea anglerfish, Phyllorhinichthys micractis (family Oneirodidae), with a histological description of the snout flaps. Copeia, 1972(2): 335-340.
Pietsch, T. W. 1973. A new genus and species of deep-sea anglerfish (Pisces: Oneirodidae) from the northern Pacific Ocean. Copeia, 1973(2): 193-199.
Pietsch, T. W. 1974a. Osteology and relationships of ceratioid anglerfishes of the family Oneirodidae, with a review of the genus Oneirodes Lütken. Nat. Hist. Mus. L. A. Co., Sci. Bull., 18, 113 pp.
Pietsch, T. W. 1974b. Systematics and distribution of ceratioid anglerfishes of the genus Lophodolos (family Oneirodidae). Breviora, 425: 1-19.
Pietsch, T. W. 1975. Systematics and distribution of ceratioid anglerfishes of the genus Chaenophryne (family Oneirodidae). Bull. Mus. Comp. Zool., 147(2): 75-100.
Pietsch, T. W. 1978. A new genus and species of deep-sea anglerfish from the eastern North Pacific Ocean, with a review of the allied genera Leptacanthichthys, Chirophryne, and Ctenochirichthys (family Oneirodidae). Nat. Hist. Mus. Los Angeles Co., Contrib. Sci., 297: 1-25.
Pietsch, T. W. 2005. Dimorphism, parasitism, and sex revisited: modes of reproduction among deep-sea ceratioid anglerfishes (Teleostei: Lophiiformes). Ichthyol. Res., 52: 207-236.
Regan, C. T. 1925. New ceratioid fishes from the N. Atlantic, the Caribbean Sea, and the Gulf of Panama, collected by the "Dana." Ann. Mag. Nat. Hist., Ser. 8, 8(62): 561-567.
Regan, C. T., and E. Trewavas. 1932. Deep-sea anglerfish (Ceratioidea). Dana Rept., 2, 113 pp.
About This Page
Theodore W. Pietsch

University of Washington, Seattle, Washington, USA
Correspondence regarding this page should be directed to Theodore W. Pietsch at
twp@u.washington.edu
and Christopher P. Kenaley at
ckenaley@u.washington.edu
Page copyright © 2005 Theodore W. Pietsch
 Page: Tree of Life
Oneirodidae. Dreamers.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Oneirodidae. Dreamers.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 05 November 2005
Citing this page:
Pietsch, Theodore W. 2005. Oneirodidae. Dreamers. Version 05 November 2005 (under construction). http://tolweb.org/Oneirodidae/22026/2005.11.05 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site