Chordata
John G. Lundberg

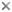
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Phylum Chordata includes the well-known vertebrates (fishes, amphibians, reptiles, birds, mammals). The vertebrates and hagfishes together comprise the taxon Craniata. The remaining chordates are the tunicates (Urochordata), lancelets (Cephalochordata), and, possibly, some odd extinct groups. With few exceptions, chordates are active animals with bilaterally symmetric bodies that are longitudinally differentiated into head, trunk and tail. The most distinctive morphological features of chordates are the notochord, nerve cord, and visceral clefts and arches.
Chordates are well represented in marine, freshwater and terrestrial habitats from the Equator to the high northern and southern latitudes. The oldest fossil chordates are of Cambrian age. The earliest is Yunnanozoon lividum from the Early Cambrian, 525 Ma (= million years ago), of China. This was just recently described and placed with the cephalochordates (Chen et al., 1995). Another possible cephalochordate is Pikaia (Nelson, 1994) from the Middle Cambrian. These fossils are highly significant because they imply the contemporary existence of the tunicates and craniates in the Early Cambrian during the so-called Cambrian Explosion of animal life. Two other extinct Cambrian taxa, the calcichordates and conodonts, are uncertainly related to other Chordata (Nelson, 1994). In the Tree of Life project, conodonts are placed as a subgroup of vertebrates.
Chordates other than craniates include entirely aquatic forms. The strictly marine Urochordata or Tunicata are commonly known as tunicates, sea squirts, and salps. There are roughly 1,600 species of urochordates; most are small solitary animals but some are colonial, organisms. Nearly all are sessile as adults but they have free-swimming, active larval forms. Urochordates are unknown as fossils. Cephalochordata are also known as amphioxus and lancelets. The group contains only about 20 species of sand-burrowing marine creatures. The Cambrian fossils Yunnanozoon and Pikaia are likely related to modern cephalochordates.
During the Ordovician Period (510 - 439 Ma) jawless or agnathan fishes appeared and diversified. These are the earliest known members of Vertebrata, the chordate subgroup that is most familiar to us. Fossils representing most major lineages of fish-like vertebrates and the earliest tetrapods (Amphibia) were in existence before the end of the Devonian Period (363 Ma). Reptile-like tetrapods originated during the Carboniferous (363 - 290 Ma), mammals differentiated before the end of the Triassic (208 Ma) and birds before the end of the Jurassic (146 Ma).
The smallest chordates (e.g. some of the tunicates and gobioid fishes) are mature at a length of about 1 cm, whereas the largest animals that have ever existed are chordates: some sauropod dinosaurs reached more than 20 m and living blue whales grow to about 30 m.
Characteristics
The notochord is an elongate, rod-like, skeletal structure dorsal to the gut tube and ventral to the nerve cord. The notochord should not be confused with the backbone or vertebral column of most adult vertebrates. The notochord appears early in embryogeny and plays an important role in promoting or organizing the embryonic development of nearby structures. In most adult chordates the notochord disappears or becomes highly modified. In some non-vertebrate chordates and fishes the notochord persists as a laterally flexible but incompressible skeletal rod that prevents telescopic collapse of the body during swimming.
The nerve cord of chordates develops dorsally in the body as a hollow tube above the notochord. In most species it differentiates in embryogeny into the brain anteriorly and spinal cord that runs through the trunk and tail. Together the brain and spinal cord are the central nervous system to which peripheral sensory and motor nerves connect.
The visceral (also called pharyngeal or gill) clefts and arches are located in the pharyngeal part of the digestive tract behind the oral cavity and anterior to the esophagus. The visceral clefts appear as several pairs of pouches that push outward from the lateral walls of the pharynx eventually to reach the surface to form the clefts. Thus the clefts are continuous, slit-like passages connecting the pharynx to the exterior. The soft and skeletal tissues between adjacent clefts are the visceral arches. The embryonic fate of the clefts and slits varies greatly depending on the taxonomic subgroup. In many of the non-vertebrate chordates, such as tunicates and cephalochordates, the clefts and arches are elaborated as straining devices concerned with capture of small food particles from water. In typical fish-like vertebrates and juvenile amphibians the walls of the pharyngeal clefts develop into gills that are organs of gas exchange between the water and blood. In adult amphibians and the amniote tetrapods (= reptiles, birds and mammals) the anteriormost cleft transforms into the auditory (Eustachian) tube and middle ear chamber, whereas the other clefts disappear after making some important contributions to glands and lymphatic tissues in the throat region. The skeleton and muscles of the visceral arches are the source of a great diversity of adult structures in the vertebrates. For example, in humans (and other mammals) visceral arch derivatives include the jaw and facial muscles, the embryonic cartilaginous skeleton of the lower jaw, the alisphenoid bone in the side wall of the braincase, the three middle ear ossicles (malleus, incus and stapes), the skeleton and some musculature of the tongue, the skeleton and muscles of the larynx, and the cartilaginous tracheal rings.
Discussion of Phylogenetic Relationships
As noted below, the relationships of some of the presumed fossil chordates is based on scant evidence and there is debate about the position of especially the calcichordates and conodonts (see references cited below and Chen et al. 1995). There is strong morphological, especially embryological, evidence for monophyly of the Urochordata, Cephalochordata and Craniata, with the latter two being sister taxa. Schaeffer (1987) details several embyological synapomorphies, in addition to those noted here, that support these same relations among and monophyly of the three living chordate groups.
- Calcichordata. Jeffries (1986) provides descriptions and comparisons and argues for the placement of calcichordates near the Chordata. Other workers believe that calcichordates are closer to echinoderms. Reconstructions of these fossil organisms include visceral (pharyngeal or gill) slits that would suggest chordate affinities, but the mineralized skeleton was composed of calcite, like echinoderms, not bone as in many chordates.
- Urochordata. Evidence that tunicates are chordates comes clearly from the larval "tadpole" stage which shows pharyngeal slits and arches, dorsal hollow nerve cord, notochord and post-anal muscular (unsegmented) tail. Adults of most members are sessile filter feeders with an expanded pharynx and, like cephalochordates and larval lampreys, with an endostyle, a mucous food trap in the pharyngeal floor that is homologous with the thyroid gland of vertebrates.
- Cephalochordata. Among the living chordates there is little doubt that lancelets are most closely related to the Craniates based on synapomorphies such as segmented axial muscles and metameric organization of the visceral (pharyngeal) arches. Uniquely, the notochord of cephalochordates extends to the tip of the snout, the gonads are segmentally organized, adults have a high number (50+) gill arches, and there is a hood-like atrium covering the pharyngeal region. The Early Cambrian fossil Yunnanozoon possesses the extended notochord and segmental gonads, but lack the atrium and increased number of gill arches.
- Craniata. Because hagfishes (Myxini) lack all traces of vertebrae, i.e. a backbone, Janvier (1981) groups the Myxini with all other vertebrates in the higher taxon Craniata (referring to the presence of a head skeleton). The taxon Vertebrata is, therefore, in a strict sense, applied to those animals known or believed to possess at least a simple backbone of neural arches. Synapomorphies of the Craniata include: presence of a cartilaginous (and often bony) head skeleton; relatively large brain plus a unique set of sensory and motor cranial nerves; nephrons as the functional excretory unit; neural crest embryonic tissue.
The traditional taxa Agnatha (jawless fishes), Ostracodermi (fossil jawless fishes) and Cyclostomata (living lampreys and hagfishes) are non-monophyletic assemblages that are no longer recommended. Details of jawless fish relationships are introduced on the Craniata and Vertebrata pages.
References
Briggs, D.E.G. 1992. Conodonts: a major extinct group added to the vertebrates. Science. 232:1295-1296.
Carroll, R. H. 1988. Vertebrate paleontology and evolution. W. H. Freeman & Co. New York.
Chen, J.Y., J. Dzik, G.D. Edgecombe, L. Ramskold and G.-Q. Zhouu. 1995. A possible Early Cambrian chordate. Nature 377:720-722.
Dehal, P., Y. Satou, R. K. Campbell, J. Chapman, B. Degnan, A. De Tomaso, B. Davidson, A. Di Gregorio, M. Gelpke, D. M. Goodstein, N. Harafuji, K. E. M. Hastings, I. Ho, K. Hotta, W. Huang, T. Kawashima, P. Lemaire, D. Martinez, I. A. Meinertzhagen, S. Necula, M. Nonaka, N. Putnam, S. Rash, H. Saiga, M. Satake, A. Terry, L. Yamada, H.-G. Wang, S. Awazu, K. Azumi, J. Boore, M. Branno, S. Chin-bow, R. DeSantis, S. Doyle, P. Francino, D. N. Keys, S. Haga, H. Hayashi, K. Hino, K. S. Imai, K. Inaba, S. Kano, K. Kobayashi, M. Kobayashi, B.-I. Lee, K. W. Makabe, C. Manohar, G. Matassi, M. Medina, Y. Mochizuki, S. Mount, T. Morishita, S. Miura, A. Nakayama, S. Nishizaka, H. Nomoto, F. Ohta, K. Oishi, I. Rigoutsos, M. Sano, A. Sasaki, Y. Sasakura, E. Shoguchi, T. Shin-i, A. Spagnuolo, D. Stainier, M. M. Suzuki, O. Tassy, N. Takatori, M. Tokuoka, K. Yagi, F. Yoshizaki, S. Wada, C. Zhang, P. D. Hyatt, F. Larimer, C. Detter, N. Doggett, T. Glavina, T. Hawkins, P. Richardson, S. Lucas, Y. Kohara, M. Levine, N. Satoh, and D. S. Rokhsar1. 2002. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 298:2157-2167.
Janvier, P. 1981. The phylogeny of the Craniata, with particular reference to the significance of fossil "agnathans." J. Vert. Paleont. 1(2):121-159.
Janvier, P. 1995. Early vertebrates. Oxford Monographs on Geology and Geophysics.
Jeffries, R.P.S. 1986. The ancestry of vertebrates. London: British Museum (Natural History).
Maisey, J.G. 1986. Heads and tails: a chordate phylogeny. Cladistics 2:201-256.
Nelson, J.S. 1994. Fishes of the World. 3rd Ed. John Wiley & Sons, New York, N.Y.
Raineri, M. 2006. Are protochordates chordates? Biological Journal of the Linnean Society 87:261-284.
Rychel, A. L., S. E. Smith, H. T. Shimamoto, and B. J. Swalla. 2006. Evolution and development of the chordates: Collagen and pharyngeal cartilage. Molecular Biology and Evolution 23:541-549.
Schaeffer, B. 1987. Deuterostome monophyly and phylogeny. Evolutionary Biology 21:179-235.
Information on the Internet
- Animal Diversity Web: Chordata
- Introduction to the Chordate. UCMP Berkeley.
Title Illustrations

Chordates include familiar vertebrates like fishes and dinosaurs, as well as less familiar marine organisms like tunicates (Ciona).
| Scientific Name | Ciona, Roeboides |
|---|---|
| Acknowledgements | Gray Museum Slide Collection (Ciona), © fish Antonio Machado. |
| Copyright |
© 1995 Marine Biological Laboratory, Woods Hole

|
About This Page
M. Laurin provided helpful comments on the text.
John G. Lundberg

The Academy of Natural Sciences, Philadelphia, Pennsylvania, USA
Page copyright © 1995 John G. Lundberg
 Page: Tree of Life
Chordata.
Authored by
John G. Lundberg.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Chordata.
Authored by
John G. Lundberg.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Citing this page:
Lundberg, John G. 1995. Chordata. Version 01 January 1995 (under construction). http://tolweb.org/Chordata/2499/1995.01.01 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site