Choanoflagellates
Choanoflagellida, collared-flagellates
Stephen Fairclough and Nicole King

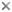
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction

Choanoflagellate cell morphology. © Stephen Fairclough
Choanoflagellates are free-living, single-cell and colony forming eukaryotes ubiquitous in aquatic environments. As their name implies, choanoflagellates (collared flagellates) have a distinctive cell morphology characterized by an ovoid or spherical cell body 3-10 µm in diameter with a single apical flagellum surrounded by a collar of 30-40 microvilli (see figure). Movement of the flagellum creates water currents that can propel free-swimming choanoflagellates through the water column and trap bacteria and detritus against the collar of microvilli where these foodstuffs are engulfed (see this animation from the University of Alberta).
Choanoflagellates are found globally in marine, brackish and freshwater environments from the Arctic to the tropics, occupying both pelagic and benthic zones. Although most sampling of choanoflagellates has occurred between 0 m and 25 m, they have been recovered from as deep as 300 m in open water (Thomsen, 1982) and 100 m under Antarctic ice sheets (Buck and Garrison, 1988). Many species are hypothesized to be cosmopolitan on a global scale [e.g., Diaphanoeca grandis has been reported from North America, Europe and Australia (OBIS)], while other species are reported to have restricted regional distributions (Thomsen, et al., 1991). Co-distributed choanoflagellate species can occupy quite different microenvironments, but in general, the factors that influence the distribution and dispersion of choanoflagellates remain to be elucidated. Irrespective of their distribution, choanoflagellates are in high abundance relative to other nanoplankton members in their communities, and there is a positive correlation between primary producers and choanoflagellate densities, supporting a model in which choanoflagellates play a pivotal role in the microbial food web and carbon cycling (Buck and Garrison, 1988).
In addition to their critical ecological roles, choanoflagellates are of particular interest to evolutionary biologists studying the origins of multicellularity in animals. As one of the closest living relatives of animals, choanoflagellates serve as a useful model for reconstructions of the last unicellular ancestor of animals.
Characteristics
In addition to the single apical flagellum surrounded by actin-filled microvilli that characterizes choanoflagellates, the internal organization of organelles in the cytoplasm is constant (Leadbeater and Thomsen, 2000). A flagellar basal body sits at the base of the apical flagellum, and a second, non-flagellar basal body rests at a right angle to the flagellar base. The nucleus occupies an apical-to-central position in the cell, and food vacuoles are positioned in the basal region of the cytoplasm (Leadbeater and Thomsen, 2000; Karpov and Leadbeater, 1998). Additionally, the cell body of many choanoflagellates is surrounded by a distinguishing extracelluar matrix or periplast. These cell coverings vary greatly in structure and composition and are used by taxonomists for classification purposes. The functional significance of the periplast is unknown, but in sessile organisms, it is thought to aid in attachment to the substrate. In planktonic organisms, there is speculation that the periplast increases drag, thereby counteracting the force generated by the flagellum and increasing feeding efficiency (Leadbeater and Kelly, 2001).
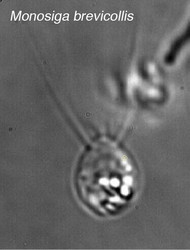
Choanoflagellates are either free-swimming in the water column or sessile, adhering to the substrate directly or through either the periplast or a thin pedicel (Leadbeater, 1983). Although choanoflagellates are thought to be strictly free-living and heterotrophic, a number of choanoflagellate relatives such as members of Ichthyosporea or Mesomycetozoa follow a parasitic or pathogenic lifestyle (Mendoza, 2002). The life histories of choanoflagellates are poorly understood. Many species are thought to be solitary; however coloniality seems to have arisen independently several times within the group and colonial species retain a solitary stage (Leadbeater, 1983).
Choanoflagellates grow vegetatively, with many species undergoing longitudinal fission (Karpov and Leadbeater, 1998); however, the reproductive life cycle of choanoflagellates remains to be elucidated. Currently, it is unclear whether there is a sexual phase to the choanoflagellate life cycle. Interestingly, some choanoflagellates can undergo encystment, which involves the retraction of the flagellum and collar and encasement in an electron dense fibrillar wall. Upon transfer to fresh media excystment occurs, though it remains to be directly observed (Leadbeater and Karpov, 2000). Further examination of the choanoflagellate life cycle will be informative about mechanisms of colony formation and attributes present before the transition to multicellularity.
Classification
Members of the order Choanoflagellida are divided into three families based upon the composition and structure of their periplast: Codosigidae, Salpingoecidae and Acanthoecidae. Members of the family Codosigidae appear to lack a periplast when examined by light microscopy, but may have a fine outer coat visible only by electron microscopy. The family Salpingoecidae consists of species whose cells are encased in a firm theca that is visible by both light and electron microscopy. The theca is a secreted covering predominately comprised of cellulose or other polysaccharides (Adl, et al., 2005). The third family of choanoflagellates, the Acanthoecidae, contains species whose cells rest in a basket-like lorica composed of siliceous ribs or “costae” (Leadbeater and Kelly, 2001; Leadbeater and Thomsen, 2000).
Discussion of Phylogenetic Relationships
The choanoflagellate families and their relationships to each other have not been tested within a phylogenetic framework. The reconstruction of the internal relationships of choanoflagellates is central to the goal of polarizing character evolution within the clade. Resolution of the internal relationships and character polarity within choanoflagellates will be informative about the character states that are ancestral within choanoflagellates and suggestive of the characteristics of the last unicellular ancestor of animals.
Relationship of Choanoflagellates to Metazoans
Dujardin, a French biologist interested in protozoan evolution, recorded the morphological similarities of choanoflagellates and sponge choanocytes and proposed the possibility of a close relationship as early as 1841 (Leadbeater and Kelly, 2001). Over the past decade, this hypothesized relationship between choanoflagellates and animals has been upheld by independent analyses of multiple unlinked sequences: 18S rDNA, nuclear protein-coding genes, and mitochondrial genomes (Steenkamp, et al., 2006; Burger, et al., 2003; Mendoza, et al., 2002; Wainright, et al., 1993). Importantly, comparisons of mitochondrial genome sequences from a choanoflagellate and three sponges confirm the placement of choanoflagellates as an outgroup to Metazoa and negate the possibility that choanoflagellates evolved from metazoans (Lavrov, et al., 2005). Finally, recent studies of genes expressed in choanoflagellates have revealed that choanoflagellates synthesize homologues of metazoan cell signaling and adhesion genes (King, 2003). Because choanoflagellates and metazoans are closely related, comparisons between the two groups promise to provide insights into the biology of their last common ancestor and the earliest events in metazoan evolution.
References
Adl, S., Simpson, A. G. B., Farmer, M. A., Adersen, R. A., Anderson, O. R., Barta, J. R., Bowser, S. S., Brugerolle, G., Fensome, R. A., Fredericq, S., James, T. Y., Karpov, S., Kugrens, P., Krug, J., Lane, C. E., Lewis, L. A., Lodge, J., Lynn, D. H., Mann, D. G., McCourt, R. M., Mendoza, L., Moestrup, Ø., Mozley-Standridge, S. E., Nerad, T. A., Shearer, C. A., Smirnov, A. V., Spiegel, F. W., and Taylor, M. F. J. R. 2005. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 52:399–451.
Bell, G. (1988) Sex and Death in Protozoa: The History of an Obsession. Cambridge : University Press.
Borchiellini, C. and Chombard, C. and Manuel, M. and Alivon, E. and Vacelet, J. and Boury-Esnault, N. (2004) Molecular phylogeny of Demospongiae: implications for classification and scenarios of character evolution. Mol. Phylogenet. Evol. , 32 pp. 823-37.
Buck, K. and Garrison, D. (1988) Distribution and abundance of choanoflagellates (Acanthoecidae) across the ice-edge zone in the Weddell Sea, Antarctica. Mar. Biol. , 98 pp. 263-269.
Burger, G. and Forget, L. and Zhu, Y. and Gray, M. and Lang, B. and (2003) Unique mitochondrial genome architecture in unicellular relatives of animals. P. Natl. Acad. Sci. USA, 100 pp. 892-897
Karpov, S. and Leadbeater, B. (1998) Cytoskeleton structure and composition in choanoflagellates. J. Eukaryot. Microbiol., 45 pp. 361–367
King, N. (2005) Choanoflagellates. Curr. Biol., 15 pp. 113-114
King, N. (2004) The unicellular ancestry of animal development. Dev. Cell., 7 pp.313-325
King, N. and Carroll, S. (2001) A receptor tyrosine kinase from choanoflagellates: molecular insights into early animal evolution. P. Natl. Acad. Sci. USA., 98 pp. 15032-15037
King, N. and Christopher, H. and Carroll, S. (2003) Evolution of key cell signaling and adhesion protein families predates animal origins. Science, 301 pp. 361-363
Lavrov, D. and Forget, L. and Kelly, M. and Lang, B. (2005) Mitochondrial genomes of two Demosponges provide insights into an early stage of animal evolution. Mol. Biol. Evol., 22 pp. 1231-1239.
Leadbeater, B. (1983) Life-History and Ultrastrucutre of a New Marine Species of Proterospongia (Choanoflagellida). J. Mar. Biol. Ass. U.K., 63 pp. 135-160
Leadbeater, B. and Karpov, S. (2000) Cyst Formation in a Freshwater Strain of the Choanoflagellate Desmarella moniliformis Kent. J. Eukaryot. Microbiol., 47 pp. 433-439
Leadbeater, B. and Kelly, M. (2001) Evolution of animals choanoflagellates and sponges. Water and Atmosphere Online. 9 pp. 9-11
Leadbeater, B. and Thomsen, H. (2000) Order choanoflagellida. In An Illustrated Guide to the Protozoa, Second Edition. (pp. 14 -38 ). Lawrence : Society of Protozoologists.
Mendoza, L. and Taylor, J. and Ajello, L. (2002) The class mesomycetozoea: a heterogeneous group of microorganisms at the animal-fungal boundary. Ann. Rev. Microbiol., 56 pp. 315-44.
Nerad, A. and Daggett, P. (1992) Cultivation of Choanoflagellates. In Protocols In Protozoology. (pp. A-20.1-A-20.4). Lawerence : Society of Protozoologists.
Ocean Biogeographic Information System. (2006). www.iobis.org
Patterson, D. (1996) Free-Living Freshwater Protozoa: A Colour Guide. Washington, D.C. : ASM Press
Reid, P. and Turley, C. and Burkhill, P. (1988) Protozoa and Their Role in Marine Processes. Berling Heidelberg : Springer-Verlag
Rokas, A. and King, N. and Finnerty, J, and Carroll, S. (2003) Conflicting phylogenetic signals at the base of the metazoan tree. Evol. & Dev., 5 pp. 346-359
Steenkamp, E. and Wright, J. and Baldauf, S. (2006) The Protistan Origins of Animals and Fungi. Mol. Bio. Evol., 23 pp. 93-106.
Thomsen, H. and Buck, K. and Chavez, F. (1991) Choanoflagellates of the central California waters: Taxonomy, morphology and species assemblages. Ophelia, 33 pp. 131-164.
Thomsen, H. (1982) Planktonic choanoflagellates from Disko Bugt, West Greenland, with a survey of the marine nanoplankton of the area. Meddelelser om Gronland, Bioscience, 8 pp. 3-36
Wainright, P. and Hinkle, G. and Sogin, M. and Stickle, S. (1993) Monophyletic origins of the metazoa: an evolutionary link with fungi. Science, 260 pp. 340-342.
Title Illustrations

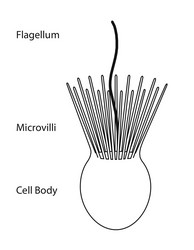
| Scientific Name | Monosiga brevicollis |
|---|---|
| Specimen Condition | Live Specimen |
| Copyright | © 2006 King Lab |
| Scientific Name | Proterospongia sp. |
|---|---|
| Specimen Condition | Live Specimen |
| Life Cycle Stage | Colony |
| Copyright | © 2006 King Lab |
About This Page
Stephen Fairclough

University of California, Berkeley, California, USA
Nicole King

University of California, Berkeley, California, USA
Page copyright © 2009 Stephen Fairclough and Nicole King
All Rights Reserved.
- First online 14 August 2006
- Content changed 14 August 2006
Citing this page:
Fairclough, Stephen and Nicole King. 2006. Choanoflagellates. Choanoflagellida, collared-flagellates. Version 14 August 2006. http://tolweb.org/Choanoflagellates/2375/2006.08.14 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site